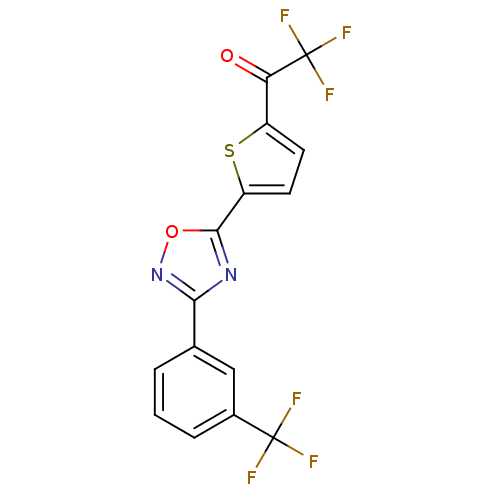
BDBM50245781 2,2,2-trifluoro-1-(5-(3-(3-(trifluoromethyl)phenyl)-1,2,4-oxadiazol-5-yl)thiophen-2-yl)ethanone::CHEMBL455395
SMILES FC(F)(F)C(=O)c1ccc(s1)-c1nc(no1)-c1cccc(c1)C(F)(F)F
InChI Key InChIKey=BNCPNGFUQKXKQH-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50245781
Found 4 hits for monomerid = 50245781
Affinity DataIC50: 320nMAssay Description:Inhibition of His-tagged HDAC4 catalytic domain expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 2.45E+3nMAssay Description:Inhibition of HDAC6More data for this Ligand-Target Pair
Affinity DataIC50: 1.75E+4nMAssay Description:Inhibition of HDAC1More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of HDAC3More data for this Ligand-Target Pair