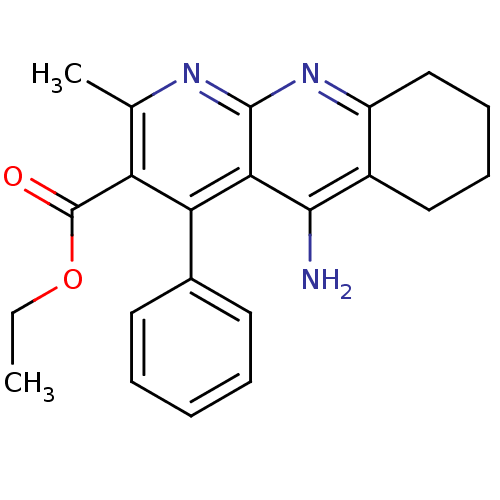
BDBM50241350 5-Amino-2-methyl-4-phenyl-6,7,8,9-tetrahydro-benzo[b][1,8]naphthyridine-3-carboxylic acid ethyl ester::CHEMBL253386::ethyl 5-amino-2-methyl-4-phenyl-6,7,8,9-tetrahydrobenzo[b][1,8]naphthyridine-3-carboxylate
SMILES CCOC(=O)c1c(C)nc2nc3CCCCc3c(N)c2c1-c1ccccc1
InChI Key InChIKey=UNCWLZOGXZCOJG-UHFFFAOYSA-N
Data 11 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50241350
Found 11 hits for monomerid = 50241350
TargetAcetylcholinesterase(Electric eel)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 822nMAssay Description:Inhibition of electric eel AChEMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 5.03E+3nMAssay Description:Inhibition of Equus caballus BuChEMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electric eel)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 820nMAssay Description:Inhibition of Electrophorus electricus AChE by spectrophotometryMore data for this Ligand-Target Pair
TargetCholinesterase(Horse)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of equine serum BuChE by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electric eel)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of electric eel AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 813nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate after 15 min by Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electric eel)
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Radicales Libres Y QuÍMica Computacional (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 820nMAssay Description:Inhibition of electric eel AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 820nMAssay Description:Inhibition of bovine erythrocytes AChE using acetylthiocholine iodide as substrate by Ellman's methodsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate by Ellman's methodsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of BuChE (unknown origin)More data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1F(Human)
Hospital Universitario De La Princesa
Curated by ChEMBL
Hospital Universitario De La Princesa
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of VGCC (unknown origin)More data for this Ligand-Target Pair