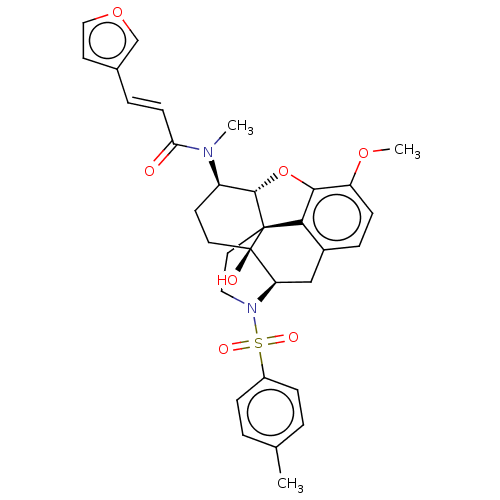
BDBM50230168 CHEMBL4079662::US10377763, Example 4
SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccc(C)cc1)ccc3OC
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50230168
Found 3 hits for monomerid = 50230168
Affinity DataKi: 5.90nMAssay Description:Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15...More data for this Ligand-Target Pair
Affinity DataIC50: 162nMAssay Description:The Chinese hamster ovary (CHO) cell lines CHOOX1R and CHOOX2R were established by modifying CHO cells to constantly express the NFAT-luciferase gene...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:The Chinese hamster ovary (CHO) cell lines CHOOX1R and CHOOX2R were established by modifying CHO cells to constantly express the NFAT-luciferase gene...More data for this Ligand-Target Pair