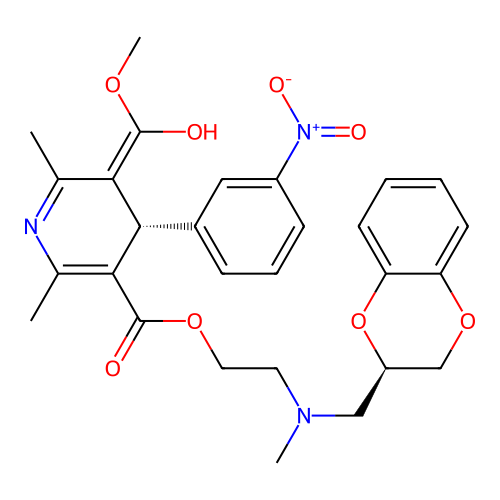
BDBM50228239 CHEMBL2093965
SMILES COC(O)=C1[C@@H](C(C(=O)OCCN(C)C[C@@H]2COc3ccccc3O2)=C(C)N=C1C)c1cccc(c1)[N+]([O-])=O
InChI Key InChIKey=JBGWBMCVMVJNTC-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50228239
Found 3 hits for monomerid = 50228239
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(Rat)
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Alpha-2-adrenolytic activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparationMore data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rat)
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparationMore data for this Ligand-Target Pair
TargetAlpha-2A/Alpha-2B/Alpha-2C adrenergic receptor(Human)
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Institut De Pharmacologie (Ua 589 Cnrs)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Alpha-2-adrenolytic activity was assessed in vitro from the ability to inhibit norepinephrine binding to guinea pig vas deferensMore data for this Ligand-Target Pair