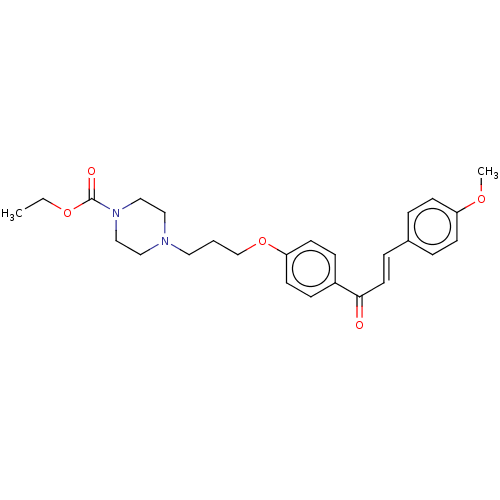
BDBM50220213 CHEMBL292275
SMILES CCOC(=O)N1CCN(CCCOc2ccc(cc2)C(=O)\C=C\c2ccc(OC)cc2)CC1
InChI Key InChIKey=BYKHDCPMWIUHPJ-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50220213
Found 3 hits for monomerid = 50220213
Affinity DataKi: 7.20nMAssay Description:Binding affinity for the rat cortical Histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 617nMAssay Description:Binding affinity for the human Histamine H1 ReceptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.76E+3nMAssay Description:Binding affinity for the human Histamine H2 receptorMore data for this Ligand-Target Pair