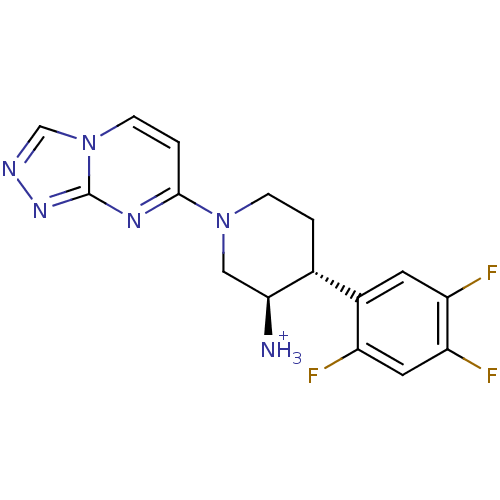
BDBM50215329 (3R,4R)-1-([1,2,4]triazolo[4,3-a]pyrimidin-7-yl)-4-(2,4,5-trifluorophenyl)piperidin-3-aminium 2,2,2-trifluoroacetate::CHEMBL231294
SMILES [NH3+][C@H]1CN(CC[C@@H]1c1cc(F)c(F)cc1F)c1ccn2cnnc2n1
InChI Key InChIKey=BVGLFSCRZQKKNX-UHFFFAOYSA-O
Data 6 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50215329
Found 6 hits for monomerid = 50215329
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of QPPMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibition of DPP9More data for this Ligand-Target Pair
Affinity DataIC50: 2.06E+4nMAssay Description:Inhibition of Cyp2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of DPP8More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human ERG potassium channelMore data for this Ligand-Target Pair