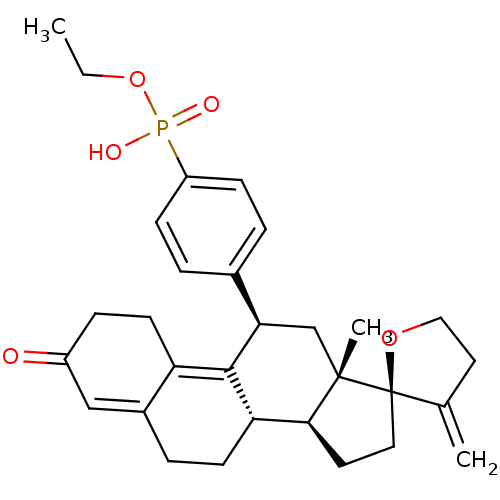
BDBM50203583 CHEMBL250949::ethoxy({4-[(2R,10'S,11'S,15'S,17'R)-15'-methyl-3-methylidene-5'-oxospiro[oxolane-2,14'-tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane]-1',6'-dien-17'-yl]phenyl})phosphinic acid
SMILES CCOP(O)(=O)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@]22OCCC2=C)[C@@H]2CCC3=CC(=O)CCC3=C12
InChI Key InChIKey=JAKSSRUUMBEEAC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50203583
Found 2 hits for monomerid = 50203583
TargetProgesterone receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at progesterone receptor expressed in human T47 cells assessed as blockade of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >3.00E+3nMAssay Description:Antagonist activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of corticoid-induced transcription by glucocortic...More data for this Ligand-Target Pair