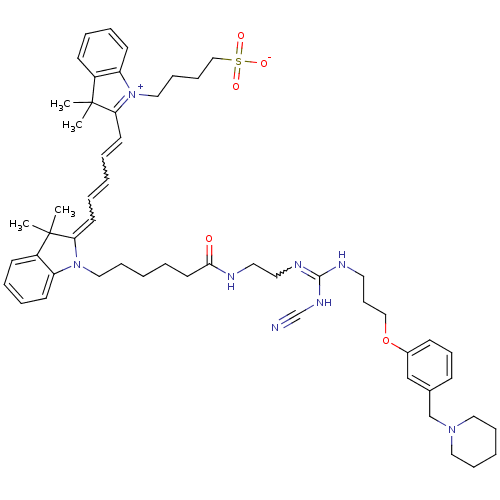
BDBM50187891 2-[(1E,3E)-5-[(2E)-1-[5-({2-[(Z)-[(cyanoamino)({3-[3-(piperidin-1-ylmethyl)phenoxy]propyl}amino)methylidene]amino]ethyl}carbamoyl)pentyl]-3,3-dimethyl-2,3-dihydro-1H-indol-2-ylidene]penta-1,3-dien-1-yl]-3,3-dimethyl-1-(4-sulfonatobutyl)-3H-indol-1-ium::CHEMBL213357
SMILES CC1(C)\C(=C/C=CC=CC2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCN=C(NCCCOc2cccc(CN3CCCCC3)c2)NC#N)c2ccccc12
InChI Key InChIKey=GFJYCIFKTFCPQN-UHFFFAOYSA-N
Data 2 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50187891
Found 2 hits for monomerid = 50187891
Affinity DataEC50: 90nMAssay Description:Agonist activity at guinea pig H2RMore data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Agonist activity at human H2RMore data for this Ligand-Target Pair