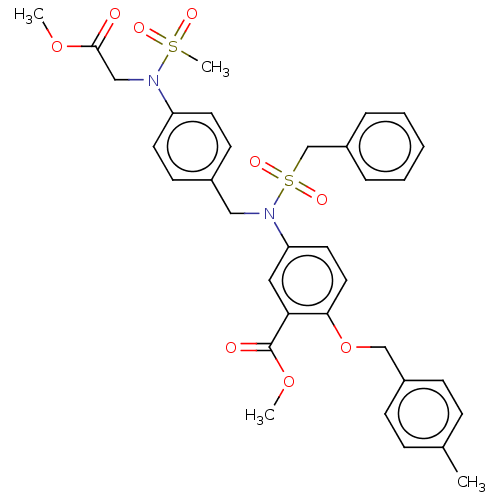
BDBM50184997 CHEMBL3822604
SMILES COC(=O)CN(c1ccc(CN(c2ccc(OCc3ccc(C)cc3)c(c2)C(=O)OC)S(=O)(=O)Cc2ccccc2)cc1)S(C)(=O)=O
InChI Key InChIKey=IPNJQCVHNPGYOX-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50184997
Found 5 hits for monomerid = 50184997
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Qilu University of Technology
Curated by ChEMBL
Qilu University of Technology
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant PTP1B (unknown origin) using pNPP as substrate incubated for 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Human)
Qilu University of Technology
Curated by ChEMBL
Qilu University of Technology
Curated by ChEMBL
Affinity DataIC50: 760nMAssay Description:Inhibition of human TCPTP using pNPP as substrate incubated for 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 6(Human)
Qilu University of Technology
Curated by ChEMBL
Qilu University of Technology
Curated by ChEMBL
Affinity DataIC50: 2.45E+3nMAssay Description:Inhibition of human SHP1 using pNPP as substrate incubated for 30 minsMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
Qilu University of Technology
Curated by ChEMBL
Qilu University of Technology
Curated by ChEMBL
Affinity DataIC50: 2.16E+3nMAssay Description:Inhibition of human SHP2 using pNPP as substrate incubated for 30 minsMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Human)
Qilu University of Technology
Curated by ChEMBL
Qilu University of Technology
Curated by ChEMBL
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of human LAR using pNPP as substrate incubated for 30 minsMore data for this Ligand-Target Pair