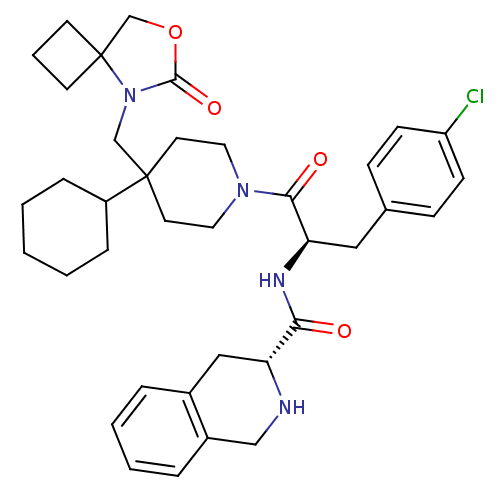
BDBM50179132 (R)-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid {(R)-1-(4-chloro-benzyl)-2-[4-cyclohexyl-4-(6-oxo-7-oxa-5-aza-spiro[3.4]oct-5-ylmethyl)-piperidin-1-yl]-2-oxo-ethyl}-amide::CHEMBL380635
SMILES Clc1ccc(C[C@@H](NC(=O)[C@H]2Cc3ccccc3CN2)C(=O)N2CCC(CN3C(=O)OCC33CCC3)(CC2)C2CCCCC2)cc1
InChI Key InChIKey=KPAXZSMXFNRRAR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50179132
Found 7 hits for monomerid = 50179132
Affinity DataIC50: 7.90nMAssay Description:Displacement of [125I]NDP-alpha-MSH from human MC4R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Activity against human MC4R by cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Displacement of [125I]NDP-alpha-MSH from human MC5R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Displacement of [125I]NDP-alpha-MSH from human MC3R expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.20E+3nMAssay Description:Activity against human MC5R by cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+3nMAssay Description:Activity against human MC1BR by cAMP accumulationMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Displacement of [125I]NDP-alpha-MSH from human MC1BR expressed in CHO cellsMore data for this Ligand-Target Pair