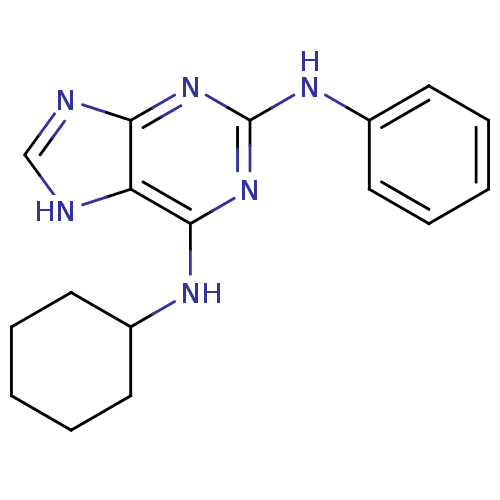
BDBM50170822 CHEMBL188592::N*6*-Cyclohexyl-N*2*-phenyl-9H-purine-2,6-diamine
SMILES C1CCC(CC1)Nc1nc(Nc2ccccc2)nc2nc[nH]c12
InChI Key InChIKey=FDFMDLSSHFEJOS-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50170822
Found 3 hits for monomerid = 50170822
TargetAdenosine receptor A3(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 51nMAssay Description:Inhibition of [125I]- AB-MECA binding to human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Percent inhibition of [3H]ZM241,385 binding to human adenosine A2a receptor expressed in CHO cells at 10 uMMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.73E+3nMAssay Description:Percent inhibition of [3H]DPCPX binding to human adenosine A1 receptor expressed in CHO cells at 10 uMMore data for this Ligand-Target Pair