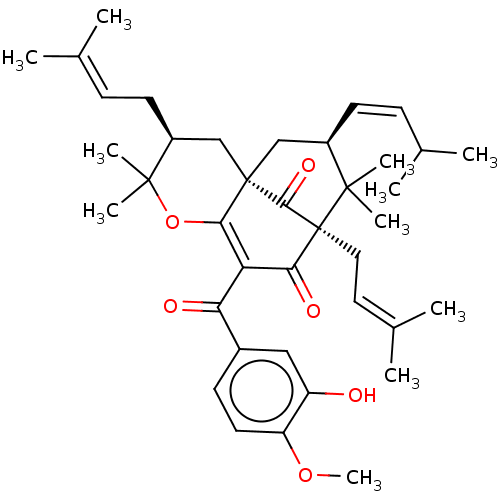
BDBM50151654 CHEMBL3775787
SMILES [#6]-[#8]-c1ccc(cc1-[#8])-[#6](=O)-[#6]-1=[#6]2-[#8]C([#6])([#6])[#6@@H](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6][C@@]22[#6]-[#6@@H](\[#6]=[#6]/[#6](-[#6])-[#6])C([#6])([#6])[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-1=O)[#6]2=O
InChI Key
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50151654
Found 1 hit for monomerid = 50151654
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of full length recombinant His6-tagged p300 (unknown origin) expressed in baculovirus infected sf21 cells using histone substrate after 10...More data for this Ligand-Target Pair