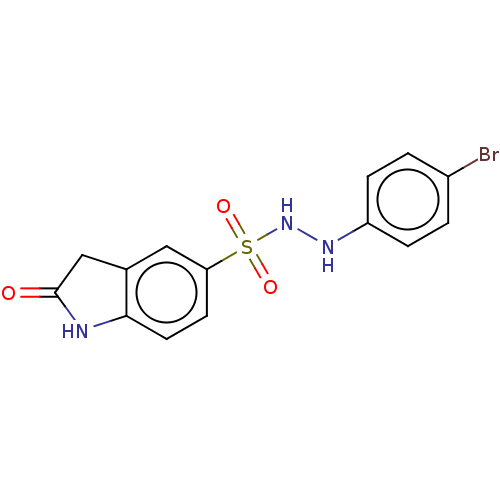
BDBM50145157 CHEMBL3763166
SMILES Brc1ccc(NNS(=O)(=O)c2ccc3NC(=O)Cc3c2)cc1
InChI Key InChIKey=LLUSLQOHYIRJHX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50145157
Found 3 hits for monomerid = 50145157
Affinity DataIC50: 36nMAssay Description:Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 68nMAssay Description:Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophanMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair