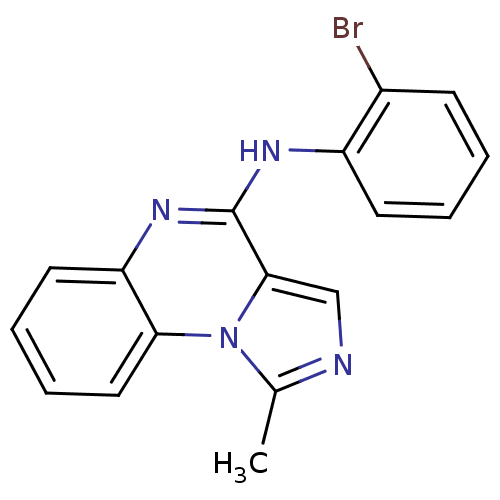
BDBM50112953 (2-Bromo-phenyl)-(1-methyl-imidazo[1,5-a]quinoxalin-4-yl)-amine::CHEMBL281056::N-(2-bromophenyl)-1-methylimidazo[1,5-a]quinoxalin-4-amine
SMILES Cc1ncc2c(Nc3ccccc3Br)nc3ccccc3n12
InChI Key InChIKey=OGXLNMDQPNBMBM-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50112953
Found 3 hits for monomerid = 50112953
TargetTyrosine-protein kinase Lck(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibitory activity against recombinant p56 Lck tyrosine kinase expressed as a His-tagged protein in insect cells using a baculovirus expression syst...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Evaluated for inhibition of human p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL