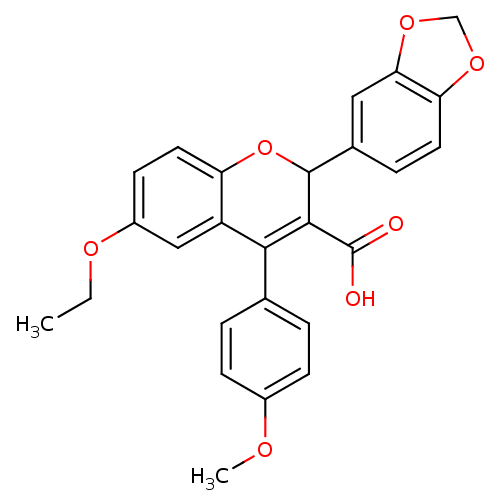
BDBM50112692 2-Benzo[1,3]dioxol-5-yl-6-ethoxy-4-(4-methoxy-phenyl)-2H-chromene-3-carboxylic acid::CHEMBL66264
SMILES CCOc1ccc2OC(C(C(O)=O)=C(c3ccc(OC)cc3)c2c1)c1ccc2OCOc2c1
InChI Key InChIKey=KMZQBJHEQFQYGF-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50112692
Found 3 hits for monomerid = 50112692
Affinity DataIC50: 400nMAssay Description:Binding affinity to endothelin B receptor measured by inhibition of [125I]ET-3 binding recombinant pig ET-B receptor expressed in COS-7 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Binding affinity for endothelin A receptor by inhibition of [125I]ET1 binding in rat aorta smooth muscle cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Ability to displace [125I]ET1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells.More data for this Ligand-Target Pair