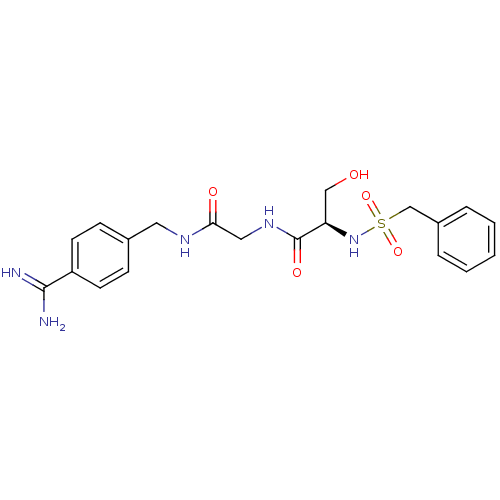
BDBM50110015 CHEMBL158936::N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AMINO(IMINO)METHYL]BENZYL}GLYCINAMIDE::N-[(4-Carbamimidoyl-benzylcarbamoyl)-methyl]-3-hydroxy-2-phenylmethanesulfonylamino-propionamide::US8476306, 6.1::US8476306, 6.2
SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1
InChI Key InChIKey=MIDRZAUOYQDRRT-UHFFFAOYSA-N
Data 15 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50110015
Found 15 hits for monomerid = 50110015
Affinity DataKi: 36nM ΔG°: -10.1kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against Plasminogen activator urokinaseMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:In vitro inhibition of plasminogen activator urokinase after a 30 min incubation period.More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Inhibition of urokinase-type plasminogen activator (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -9.40kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against Plasminogen activator urokinaseMore data for this Ligand-Target Pair
Affinity DataKi: 150nM ΔG°: -9.27kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:In vitro inhibition of trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 290nM ΔG°: -8.88kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against TrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMAssay Description:In vitro inhibition of Coagulation factor Xa.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nM ΔG°: -6.74kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against PlasminMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:In vitro inhibition of Plasmin.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of plasmin (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nM ΔG°: -6.64kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against ThrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:In vitro inhibition of thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nM ΔG°: -6.19kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against PlasminMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+4nM ΔG°: -5.95kcal/moleT: 2°CAssay Description:Inhibition constant of the compound against ThrombinMore data for this Ligand-Target Pair