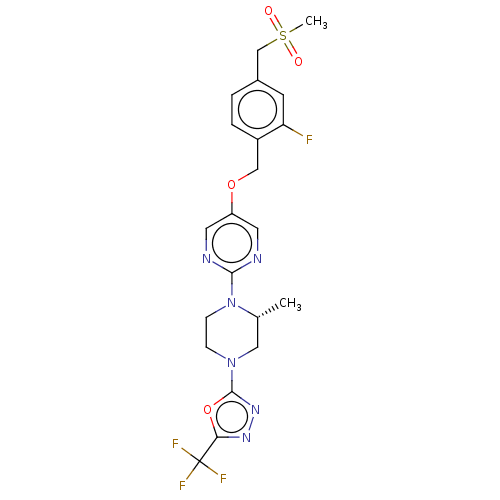
BDBM50103556 CHEMBL3358001
SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F
InChI Key InChIKey=IXIWGIOCETWRTM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50103556
Found 3 hits for monomerid = 50103556
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by patch clamp based electrophysiology methodMore data for this Ligand-Target Pair
Affinity DataEC50: 249nMAssay Description:Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 27nMAssay Description:Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assayMore data for this Ligand-Target Pair