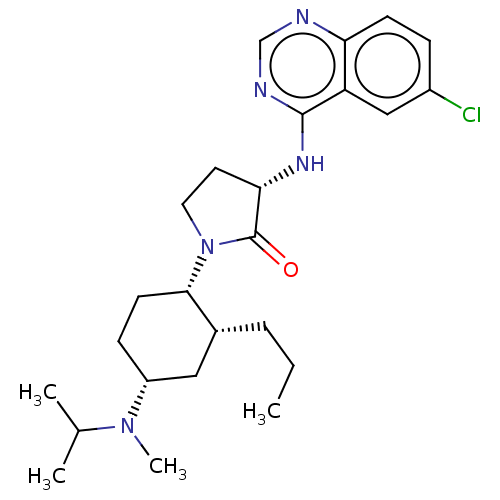
BDBM50089363 CHEMBL3577944
SMILES CCC[C@@H]1C[C@@H](CC[C@@H]1N1CC[C@H](Nc2ncnc3ccc(Cl)cc23)C1=O)N(C)C(C)C
InChI Key InChIKey=DEXJQUDODNDJEZ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50089363
Found 3 hits for monomerid = 50089363
Affinity DataIC50: 2.20nMT: 2°CAssay Description:Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperatureMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of [125I]MCP1-beta binding to CCR5 in human HT1080 cell membranes incubated for 4 to 6 hrsMore data for this Ligand-Target Pair