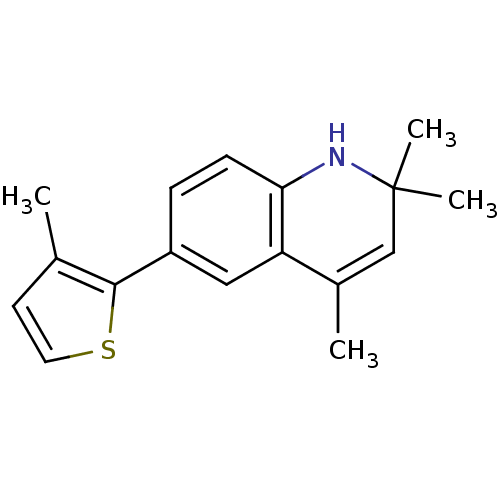
BDBM50086536 2,2,4-Trimethyl-6-(3-methyl-thiophen-2-yl)-1,2-dihydro-quinoline::2,2,4-trimethyl-6-(3-methylthiophen-2-yl)-1,2-dihydroquinoline::CHEMBL133794
SMILES Cc1ccsc1-c1ccc2NC(C)(C)C=C(C)c2c1
InChI Key InChIKey=KDEPJXRAESNIQG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50086536
Found 4 hits for monomerid = 50086536
Affinity DataKi: 25nMAssay Description:Displacement of [3H]progesterone from Progesterone receptorMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding affinity against human progesterone receptor (hPR) in a competitive binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 105nMAssay Description:Antagonist activity against human progesterone receptor (hPR) in T47D human breast cancer cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 232nMAssay Description:Antagonist activity against human progesterone receptor (hPR) using cotransfection assay in CV-1 cellsMore data for this Ligand-Target Pair