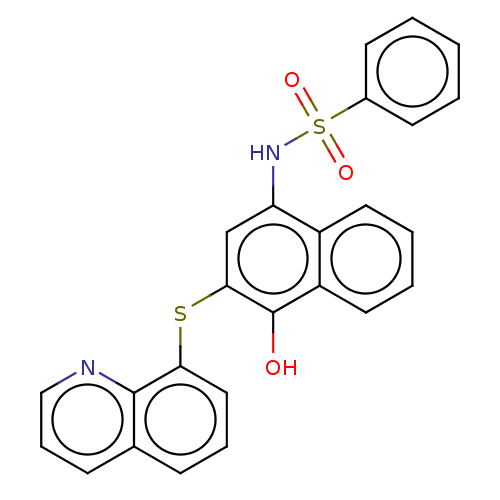
BDBM50068236 CHEMBL1576791
SMILES Oc1c(Sc2cccc3cccnc23)cc(NS(=O)(=O)c2ccccc2)c2ccccc12
InChI Key InChIKey=PQIKPRFFZWZBIW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50068236
Found 6 hits for monomerid = 50068236
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of Vps75-stimulated recombinant Saccharomyces cerevisiae histone acetyltransferase Rtt109 using Asf1-dH3-H4 as substrate assessed as coenz...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of Vps75-stimulated recombinant Saccharomyces cerevisiae histone acetyltransferase Rtt109 using Asf1-dH3-H4 as substrate assessed as coenz...More data for this Ligand-Target Pair
Affinity DataKi: 1.56E+4nMAssay Description:Displacement of [3H]LSD from human cloned 5-HT6R expressed in HEK293 cells at 0.1 and 1 uMMore data for this Ligand-Target Pair
Affinity DataKi: 2.37E+4nMAssay Description:Displacement of [3H]-8-OH-DPAT from human cloned 5-HT1A receptor expressed in HEK293 cells at 0.1 and 1 uMMore data for this Ligand-Target Pair
Affinity DataKi: 2.54E+4nMAssay Description:Displacement of [3H]-5-CT from human cloned 5-HT7R expressed in HEK293 cells at 0.1 and 1 uMMore data for this Ligand-Target Pair
Affinity DataKi: 3.42E+4nMAssay Description:Displacement of [3H]-ketanserin from human cloned 5-HT2A receptor expressed in CHO-K1 cells at 0.1 and 1 uMMore data for this Ligand-Target Pair