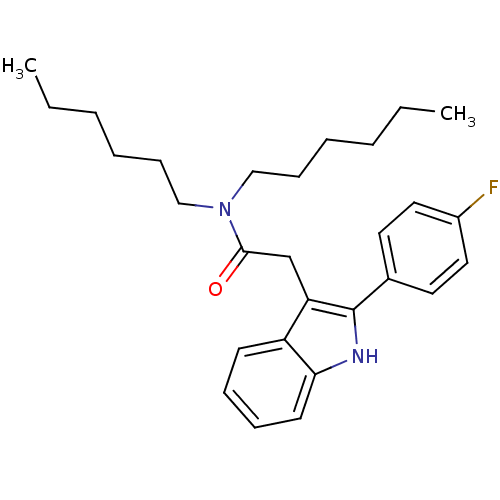
BDBM50045877 2-(2-(4-fluorophenyl)-1H-indol-3-yl)-N,N-dihexylacetamide::2-[2-(4-Fluoro-phenyl)-1H-indol-3-yl]-N,N-dihexyl-acetamide::CHEMBL63154::[N,N-di-n-hexyl-2-(4-fluorophenyl) indole-3-acetamide]
SMILES CCCCCCN(CCCCCC)C(=O)Cc1c([nH]c2ccccc12)-c1ccc(F)cc1
InChI Key InChIKey=VUWXAQFLTSBUDB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50045877
Found 14 hits for monomerid = 50045877
Affinity DataKi: 3.30nMAssay Description:Inhibition of TSPO (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 4nMAssay Description:Binding affinity to human PBRMore data for this Ligand-Target Pair
Affinity DataKi: 4.40nMAssay Description:Binding affinity against mitochondrial DBI complex (peripheral benzodiazepine receptor) using primary cultures of glial cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Displacement of [3H]PK11195 from TSPO in rat cortexMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of [3H]PK11195 binding from peripheral benzodiazepine receptor in C6 rat glioma cell lineMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of [3H]PK11195 binding from peripheral benzodiazepine receptor in MA-10 mouse tumor Leydig cellsMore data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor/Alpha-1B adrenergic receptor/Alpha-1D adrenergic receptor/Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(Rat)
Mayo Foundation
Curated by ChEMBL
Mayo Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity by measuring its ability to displace [3H]WB-4101 from alpha-adrenergic receptor in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity by measuring its ability to displace [125I]pindolol from beta-adrenergic receptor in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity by measuring its ability to displace [3H]L-365260 from cholecystokinin receptor in rat brainMore data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor/Alpha-1B adrenergic receptor/Alpha-1D adrenergic receptor/Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(Rat)
Mayo Foundation
Curated by ChEMBL
Mayo Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity by measuring its ability to displace [3H]clonidine from alpha-adrenergic receptor in rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rat)
Mayo Foundation
Curated by ChEMBL
Mayo Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Tested for inhibitory activity by measuring its ability to displace [3H]3-PPP from opiate receptor in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity, measured by displacement [3H]CP-55940 from cannabinoid receptor (CB1) in rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity by measuring its ability to displace [3H]ketanserin from serotonin receptor in rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1(Rat)
Mayo Foundation
Curated by ChEMBL
Mayo Foundation
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of [3H]naxolone binding to opiate receptors in rat brainMore data for this Ligand-Target Pair