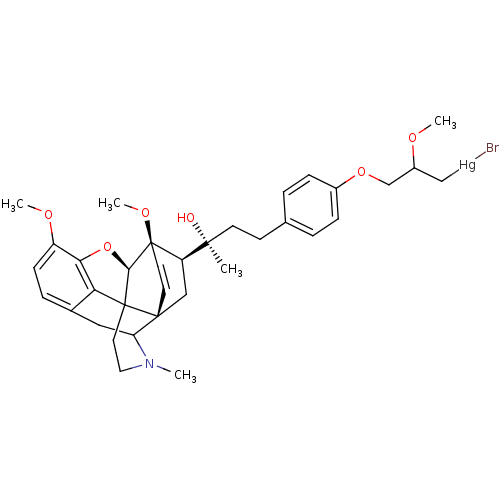
BDBM50021253 7alpha-[(1R)-1-Hydroxy1-methyl-3-[4-[3-(bromomercurio)-2-methoxypropoxy]phenyl]-6,14-endo-ethenotetrahydrothebaine]::CHEMBL58589
SMILES COC(COc1ccc(CC[C@](C)(O)[C@H]2C[C@]34C=C[C@]2(OC)[C@@H]2Oc5c6c(CC3N(C)CCC426)ccc5OC)cc1)C[Hg]Br
InChI Key InChIKey=FLMYZKLRQCJYRQ-UHFFFAOYSA-M
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50021253
Found 2 hits for monomerid = 50021253
Affinity DataIC50: 250nMAssay Description:Evaluated for the inhibition of [3H]DADLE binding to Opioid receptor delta 1 in rat brain homogenates.More data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:Evaluated for the inhibition of [3H]naltrexone binding to Opioid receptor mu 1 in rat brain homogenates.More data for this Ligand-Target Pair