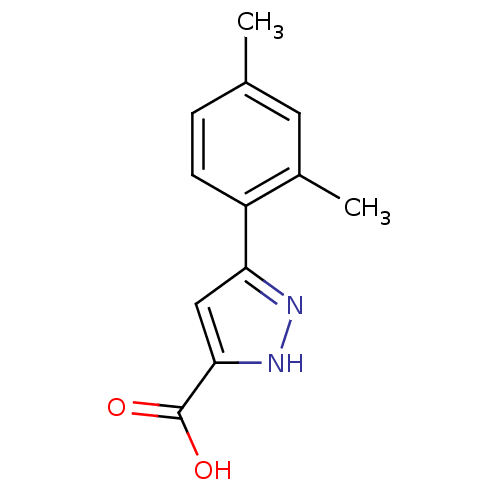
BDBM45837 3-(2,4-dimethylphenyl)-1H-pyrazole-5-carboxylic acid::MLS-0091993.0001::cid_4274816
SMILES Cc1ccc(-c2cc([nH]n2)C(O)=O)c(C)c1
InChI Key InChIKey=XSEUPOAEAAAQGG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 45837
Found 5 hits for monomerid = 45837
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.34E+3nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase 9 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase 1 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase 2 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of human recombinant carbonic anhydrase 12 expressed in Escherichia coli preincubated for 15 mins by stopped flow CO2 hydration methodMore data for this Ligand-Target Pair