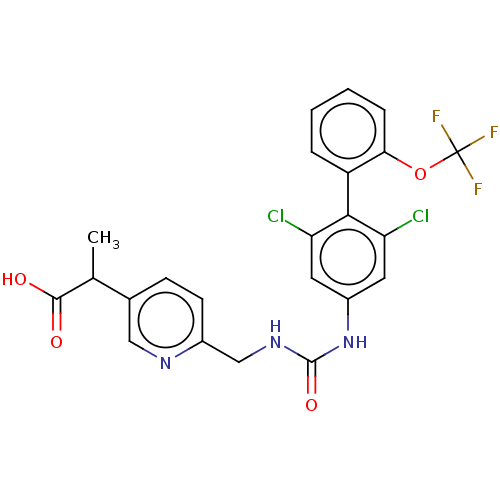
BDBM441844 US10647665, Example 1-30::US10851050, Example I-30
SMILES CC(C(O)=O)c1ccc(CNC(=O)Nc2cc(Cl)c(c(Cl)c2)-c2ccccc2OC(F)(F)F)nc1
InChI Key InChIKey=BDJXHHMHODULKW-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 441844
Found 3 hits for monomerid = 441844
Affinity DataIC50: 2.75E+3nMAssay Description:1. Preparation of RORgamma Buffer Solution10 mL of DTT and 100 mL buffer solution were gently mixed together and ready to use.2. Preparation of Compo...More data for this Ligand-Target Pair
Affinity DataIC50: 2.75E+3nMAssay Description:The inhibitory activity of compounds on RORγ receptor was determined by fluorescence resonance energy transfer (FRET) experiments. The inhibitor...More data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+3nMAssay Description:Inverse agonist activity at human RORgammat assessed as inhibition of N-terminal biotinylated co-activator SRC1 recruitment measured after 60 mins by...More data for this Ligand-Target Pair