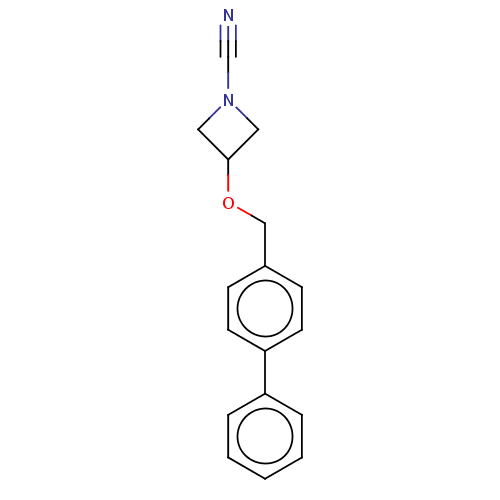
BDBM393363 3-([1,1'-Biphenyl]-4-ylmethoxy)azetidine-1-carbonitrile::US9963444, Example 64
SMILES N#CN1CC(C1)OCc1ccc(cc1)-c1ccccc1
InChI Key InChIKey=ZSTZNGPOKKKVCT-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 393363
Found 3 hits for monomerid = 393363
Affinity DataIC50: 2.80nMAssay Description:Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of NAAA (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:In order to have an assay method more conducive to high-throughput screening than those published for measuring the NAE hydrolyzing activity of NAAA,...More data for this Ligand-Target Pair