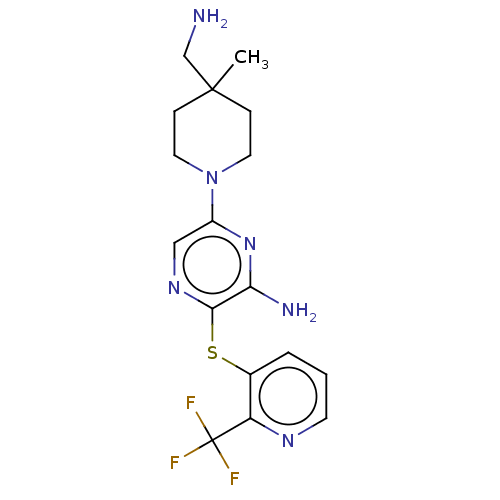
BDBM392315 6-(4-(aminomethyl)-4-methylpiperidin-1-yl)-3-((2-(trifluoromethyl)pyridin-3-yl)thio)pyrazin-2-amine::US10301278, Example 29::US12053470, Compound 4
SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccnc2C(F)(F)F)c(N)n1
InChI Key InChIKey=JBLYMVJAFCDNOA-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 392315
Found 5 hits for monomerid = 392315
Affinity DataIC50: 29nMAssay Description:SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY...More data for this Ligand-Target Pair
Affinity DataIC50: 187nMAssay Description:Allosteric inhibition of human SHP2 in human KYSE-520 cells assessed as reduction in ERK1/2 phosphorylation by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+4nMAssay Description:Inhibition of human ERG by Q-patch assayMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:More specifically, the phosphatase reactions were performed at room temperature in 384-well black polystyrene plate, flat bottom, low flange, non-bin...More data for this Ligand-Target Pair