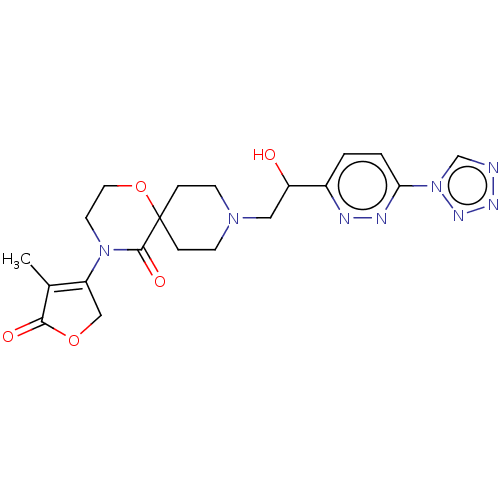
BDBM363489 9-(2-(6-(1H-tetrazol-1- yl)pyridazin-3-yl)-2- hydroxyethyl)-4-(4-methyl-5- oxo-2,5-dihydrofuran-3-yl)-1- oxa-4,9-diazaspiro[5.5]undecan- 5-one (single diastereomer, absolute stereochemistry not established, but opposite from 45B)::US9850245, Example 45A::US9850245, Example 45B
SMILES CC1=C(COC1=O)N1CCOC2(CCN(CC(O)c3ccc(nn3)-n3cnnn3)CC2)C1=O
InChI Key InChIKey=OJNVZFGKXUEICD-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 363489
Found 2 hits for monomerid = 363489
Affinity DataIC50: 15nMAssay Description:Blocking of Kir1.1 (ROMK1) currents was examined by whole cell voltage clamp (Hamill et. al. Pfluegers Archives 391:85-100 (1981)) using the IonWorks...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Blocking of Kir1.1 (ROMK1) currents was examined by whole cell voltage clamp (Hamill et. al. Pfluegers Archives 391:85-100 (1981)) using the IonWorks...More data for this Ligand-Target Pair