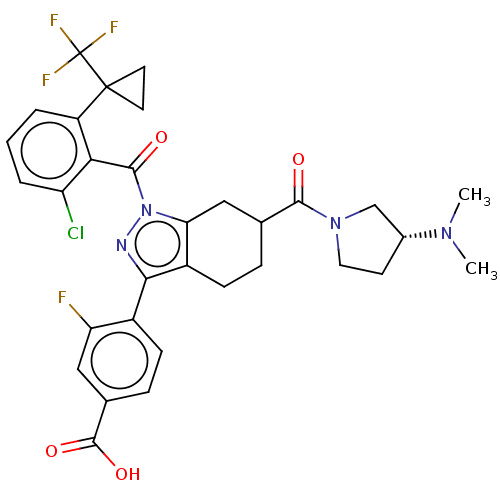
BDBM359541 4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)cyclo- propyl]phenyl}car- bonyl)-6-{[(3R)-3- (dimethylamino) pyrrolidin-1- yl]carbonyl}-4,5,6,7- tetrahydro-1H- indazol-3-yl]-3- fluorobenzoic acid::US10221142, Example 21G::US10221142, Example 21H
SMILES CN(C)[C@@H]1CCN(C1)C(=O)C1CCc2c(C1)n(nc2-c1ccc(cc1F)C(O)=O)C(=O)c1c(Cl)cccc1C1(CC1)C(F)(F)F
InChI Key InChIKey=OZXSOFDEDGPEOK-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 359541
Found 2 hits for monomerid = 359541
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair