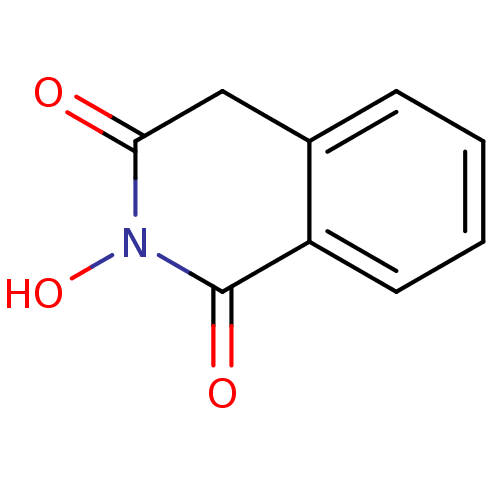
BDBM33410 CHEMBL16755::N-hydroxyisoquinolinedione, 2
SMILES c1ccc2c(c1)CC(=O)N(C2=O)O
InChI Key InChIKey=ZXAICCBFIBBVAR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 33410
Found 15 hits for monomerid = 33410
Affinity DataIC50: 135nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of reconstituted HIV1 RNase H using RNA/DNA duplex substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in RNA 5' end directed clea...More data for this Ligand-Target Pair
TargetPolymerase acidic protein(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
University of Tennessee Health Science Center
Curated by ChEMBL
University of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataKi: 850nMAssay Description:Binding affinity to PA N-terminal domain (Competitive)More data for this Ligand-Target Pair
Affinity DataIC50: 860nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase RNasH activityMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in DNA 3' end directed clea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of HIV1 recombinant reverse transcriptase associated catalytically active RNase H domain assessed as reduction in internal cleavage using ...More data for this Ligand-Target Pair
TargetPolymerase acidic protein(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
University of Tennessee Health Science Center
Curated by ChEMBL
University of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataEC50: 3.22E+3nMAssay Description:Plaque growth inhibitionMore data for this Ligand-Target Pair
Affinity DataIC50: 6.32E+3nMAssay Description:Inhibition of HIV1 integraseMore data for this Ligand-Target Pair
TargetPolymerase acidic protein(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
University of Tennessee Health Science Center
Curated by ChEMBL
University of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibitory concentration against cap-dependent endonuclease activity of influenza virus RNPMore data for this Ligand-Target Pair
TargetPolymerase acidic protein(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...)
University of Tennessee Health Science Center
Curated by ChEMBL
University of Tennessee Health Science Center
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of influenza A virus (A/PR/8/34) PA endonuclease using ALMV cap1 primer RNA as substrate after 90 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of HIV1 recombinant reverse transcriptase polymerase activity using [3H]TTP and poly(rA)-oligo(dT)16 substrate incubated for 20 mins by li...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+5nMAssay Description:Commercially available 2-Hydroxyisoquinoline-1,3(2H,4H)-dione (CAS No: 6890-08-0), a well-known scaffold backbone used to develop human immunodeficie...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+6nMAssay Description:Inhibition of HIV1 integrase strand transfer activityMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)