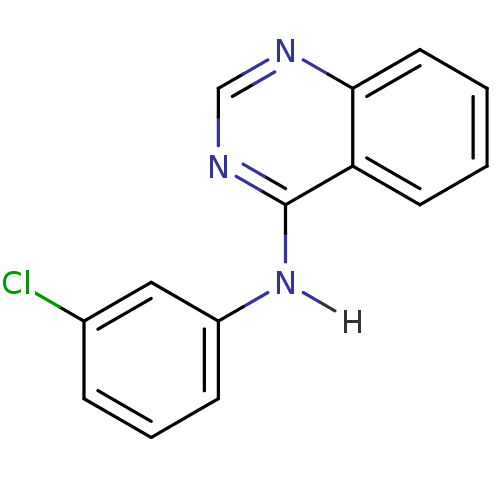
BDBM3263 4-Anilino quinazoline deriv. 14::CHEMBL329672::CHEMBL555321::N-(3-chlorophenyl)quinazolin-4-amine
SMILES Clc1cccc(Nc2ncnc3ccccc23)c1
InChI Key InChIKey=ZKKVUIPXPPDIRD-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 3263
Found 8 hits for monomerid = 3263
Affinity DataIC50: 11nMAssay Description:Inhibition of liver delta-5 desaturase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation, 0.05-0.10More data for this Ligand-Target Pair
Affinity DataIC50: 4.16E+3nMpH: 7.6 T: 2°CAssay Description:The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate.More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of p56lck kinase autophosphorylation in Jurkat cellsMore data for this Ligand-Target Pair