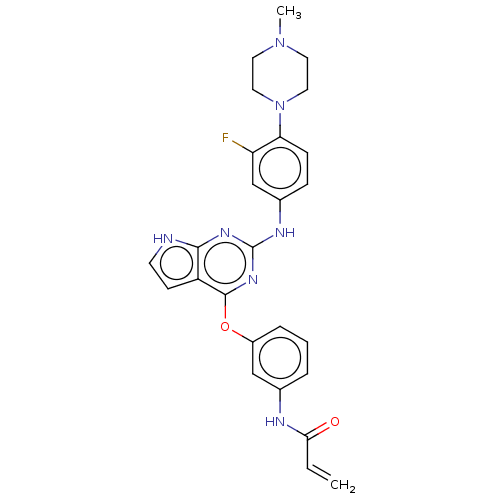
BDBM294480 N-(3-((2-((3-fluoro-4-(4-methylpiperazin-1- yl)phenyl)amino)-7H-pyrrolo[2,3-d]- pyrimidin-4-yl)oxy)phenyl)acrylamide::US9586965, Cpd 3
SMILES CN1CCN(CC1)c2ccc(cc2F)Nc3nc4c(cc[nH]4)c(n3)Oc5cccc(c5)NC(=O)C=C
InChI Key InChIKey=UOFYSRZSLXWIQB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 294480
Found 13 hits for monomerid = 294480
Affinity DataIC50: 0.0911nMpH: 7.5 T: 2°CAssay Description:Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.170nMAssay Description:Inhibition of wild type EGFR L858R mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 mins by m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.399nMpH: 7.5 T: 2°CAssay Description:Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Inhibition of wild type EGFR C797S mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 mins by m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of wild type EGFR L858R/T790M mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 min...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of wild type EGFR Del E746/A750 mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 m...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of wild type EGFR (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 mins by mobility shift...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 177nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 279nMAssay Description:Inhibition of wild type EGFR L858R/T790M/C797S mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after ...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Sichuan University
Curated by ChEMBL
Sichuan University
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibition of LRRK2 G2019S mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 501nMpH: 7.5 T: 2°CAssay Description:Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+3nMpH: 7.5 T: 2°CAssay Description:Briefly, specific kinase/substrate pairs along with required cofactors were prepared in reaction buffer. Compounds were delivered into the reaction, ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR T790M mutant (unknown origin) pretreated with substrate for 10 mins followed by ATP addition measured after 60 mins by m...More data for this Ligand-Target Pair