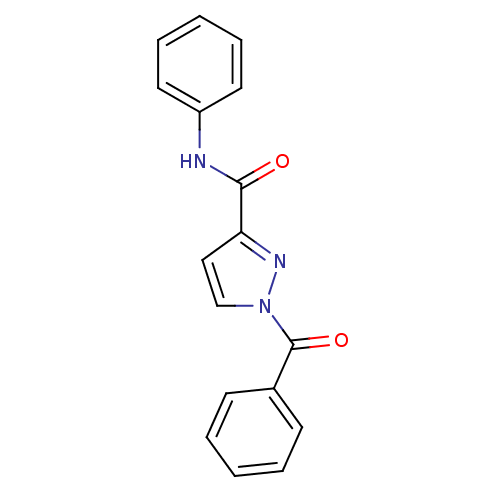
BDBM23704 1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide::N-Benzoylpyrazole deriv., 6
SMILES O=C(Nc1ccccc1)c1ccn(n1)C(=O)c1ccccc1
InChI Key InChIKey=ZYYMCOZRPAHLRN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 23704
Found 7 hits for monomerid = 23704
Affinity DataKi: 39nM ΔG°: -10.1kcal/molepH: 7.5 T: 2°CAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+4nMAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair
Affinity DataAssay Description:HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer...More data for this Ligand-Target Pair