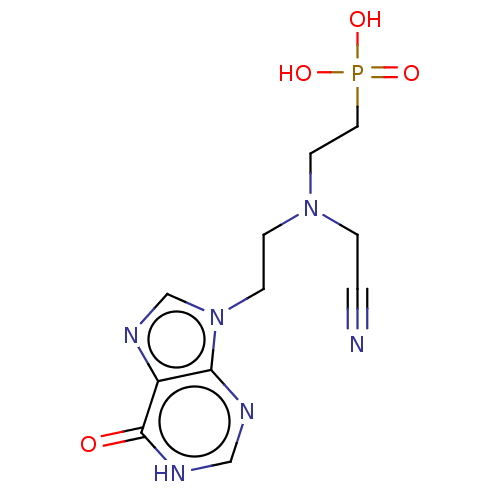
BDBM194495 BDBM50392250::US9200020, Table 3 compound 4
SMILES OP(O)(=O)CCN(CCn1cnc2c1nc[nH]c2=O)CC#N
InChI Key InChIKey=SGDYHKAMNIRJKE-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 194495
Found 4 hits for monomerid = 194495
TargetHypoxanthine-guanine phosphoribosyltransferase(Human)
Academy of Sciences of The Czech Republic
Curated by ChEMBL
Academy of Sciences of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 3.50E+4nMAssay Description:Inhibition of human HGPRT using Prib-PP as substrate by spectrophotometric assay in presence of guanineMore data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Human)
Academy of Sciences of The Czech Republic
Curated by ChEMBL
Academy of Sciences of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 3.50E+4nM ΔG°: -6.32kcal/moleT: 2°CAssay Description:The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitro ...More data for this Ligand-Target Pair
Affinity DataKi: 4.70E+4nM ΔG°: -6.14kcal/moleT: 2°CAssay Description:The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitro ...More data for this Ligand-Target Pair
TargetHypoxanthine-guanine-xanthine phosphoribosyltransferase(Plasmodium falciparum)
The University of Queensland
US Patent
The University of Queensland
US Patent
Affinity DataKi: >2.50E+5nM ΔG°: >-5.11kcal/moleT: 2°CAssay Description:The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitro ...More data for this Ligand-Target Pair