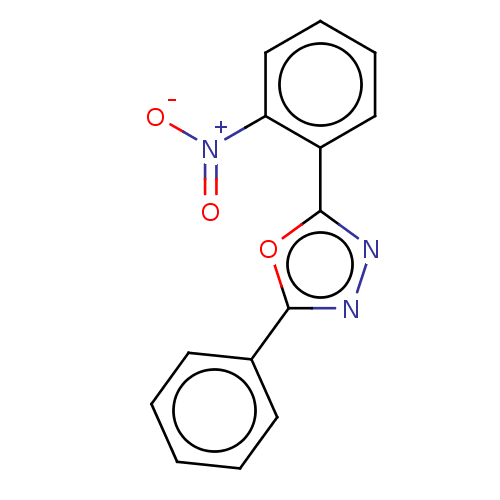
BDBM192667 2-phenyl-5-(2-nitrophenyl)-1,3,4-oxadiazole (2i)
SMILES [O-][N+](=O)c1ccccc1-c1nnc(o1)-c1ccccc1
InChI Key InChIKey=LIJMRRGETTVLFW-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 192667
Found 3 hits for monomerid = 192667
Affinity DataKi: 57nM ΔG°: -10.3kcal/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 790nM ΔG°: -8.65kcal/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 6.69E+3nM ΔG°: -7.34kcal/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair