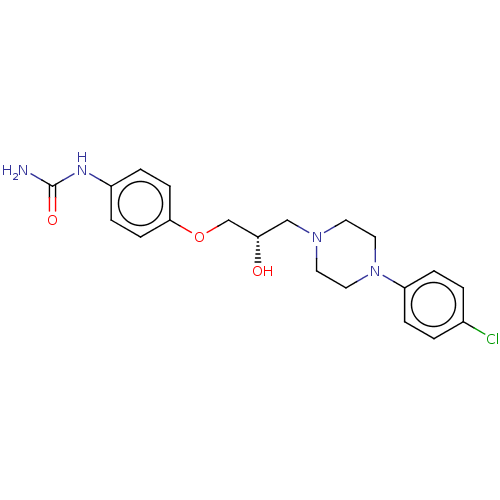
BDBM168619 US9079852, Table F, Compound 4
SMILES NC(=O)Nc1ccc(OC[C@@H](O)CN2CCN(CC2)c2ccc(Cl)cc2)cc1
InChI Key InChIKey=GSOWCEUNQPJIFR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 168619
Found 4 hits for monomerid = 168619
Affinity DataIC50: 46nMAssay Description:The GluN2B potency and pH dependence of NP10679, NP10309, and other compounds in Tables 1 and 2 were evaluated on human GluN1-la/GluN2B receptors (he...More data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ...More data for this Ligand-Target Pair
Affinity DataIC50: 452nMAssay Description:The GluN2B potency and pH dependence of NP10679, NP10309, and other compounds in Tables 1 and 2 were evaluated on human GluN1-la/GluN2B receptors (he...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole...More data for this Ligand-Target Pair