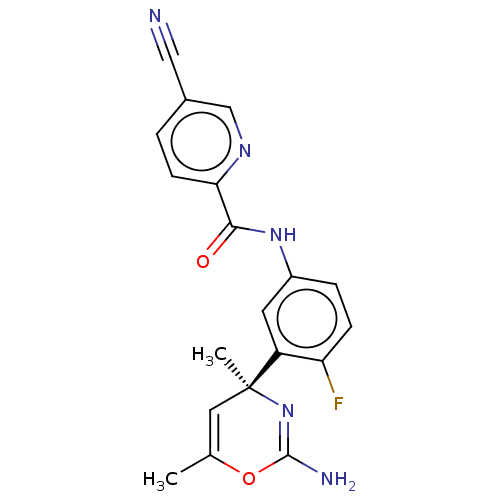
BDBM153632 US8999980, I-54
SMILES CC1=C[C@](C)(N=C(N)O1)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F
InChI Key InChIKey=LLUIVDBCIQNSNG-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 153632
Found 3 hits for monomerid = 153632
Affinity DataIC50: 4.40nMAssay Description:Inhibition of BACE1 in human SH-SY5Y cells transfected with human wild type beta-APP assessed as reduction in amyloid beta (1 to 40) levels after 24 ...More data for this Ligand-Target Pair
Affinity DataIC50: 66nMAssay Description:Inhibition of human recombinant BACE1 (43 to 545 residues) expressed in Escherichia coli BL21(DE3) using APP Swedish Lys-Met/Asn-Leu mutant as substr...More data for this Ligand-Target Pair
Affinity DataIC50: 97nMpH: 5.0 T: 2°CAssay Description:48.5 .mu.L of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=.epsilon.-amino-n-capronic acid, Eu=Europium cryptate) was added to each well...More data for this Ligand-Target Pair