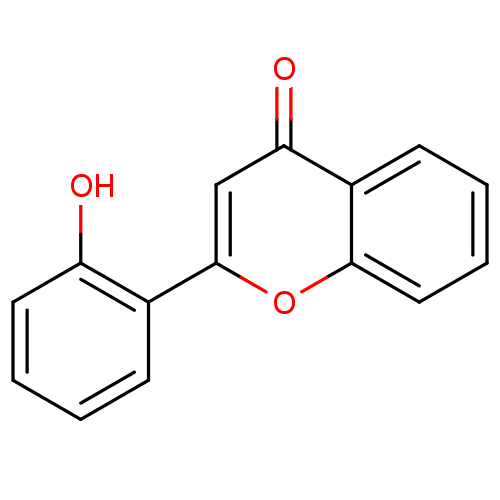
BDBM150758 2-(2-Hydroxyphenyl)-4H-chromen-4-one (3d)::US9271961, 2'-Hydroxyflavone
SMILES Oc1ccccc1-c1cc(=O)c2ccccc2o1
InChI Key InChIKey=ZZLQHXCRRMUGQJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 150758
Found 7 hits for monomerid = 150758
Affinity DataIC50: 300nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataKi: 520nMAssay Description:The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at AR in human LNCAP cells assessed as reduction in DHT-induced transcriptional activation after 24 hrs by luciferase reporter ge...More data for this Ligand-Target Pair
Affinity DataKi: 5.98E+3nMAssay Description:The activities of recombinant hMAO-A and hMAO-B were determined using p-tyramine as common substrate and calculated as 0.18 +/- 0.01 nmol/mg/min (n =...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Antagonist activity at AR in human LNCAP cells assessed as reduction in DHT-induced cell growth treated for every 2 days for 4 days by hemocytometryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.71E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair