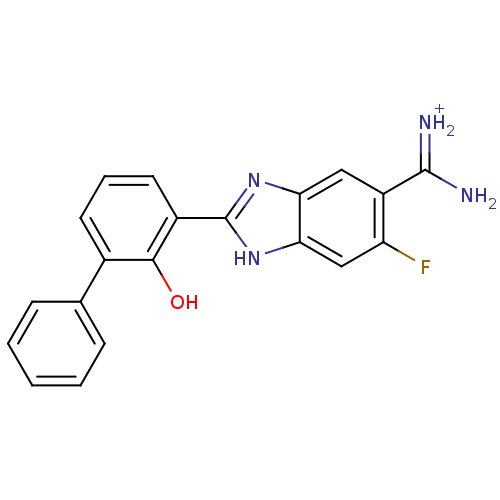
BDBM14147 APC-10818::{amino[6-fluoro-2-(2-hydroxy-3-phenylphenyl)-1H-1,3-benzodiazol-5-yl]methylidene}azanium
SMILES NC(=[NH2+])c1cc2nc([nH]c2cc1F)-c1cccc(-c2ccccc2)c1O
InChI Key InChIKey=QXRGLPOCPFPVHS-UHFFFAOYSA-O
Data 18 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 14147
Found 18 hits for monomerid = 14147
Affinity DataKi: 250nM ΔG°: -8.91kcal/molepH: 8.2 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 550nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 590nM ΔG°: -8.41kcal/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 3.50E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 4.70E+4nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+5nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: >4.50E+5nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair