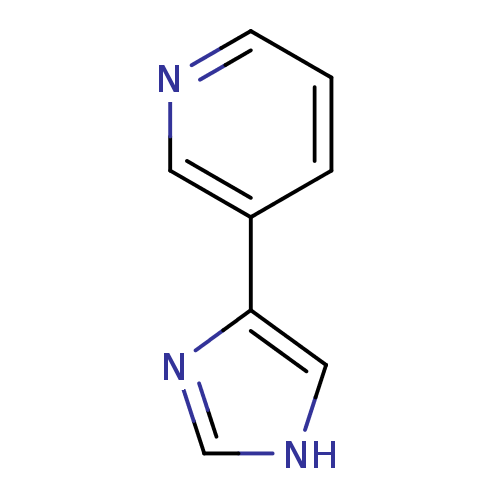
BDBM12357 3-(1H-imidazol-4-yl)pyridine::CHEMBL178516::JMC514968 Compound 7::US8609708, 14::US8609708,14::nicotine 3-heteroaromatic analogue 10
SMILES c1nc(c[nH]1)-c1cccnc1
InChI Key InChIKey=YFOKBFRTGLSZLU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 12357
Found 16 hits for monomerid = 12357
Affinity DataKi: 250nMAssay Description:Effect on coumarin 7-hydroxylation by human Cytochrome P-450 2A6More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibitory concentration value against human cytochrome P-450 2A6More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.55E+4nMAssay Description:Inhibitory concentration value against human cytochrome P-450 2E1More data for this Ligand-Target Pair
Affinity DataIC50: 4.18E+4nMAssay Description:Inhibitory concentration against human cytochrome P-450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 5.35E+4nMAssay Description:Inhibitory concentration value against human cytochrome P-450 2C19More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+4nMAssay Description:To measure CYP2A6 activity, coumarin 7-hydroxylation was determined. The formation of the coumarin metabolite, 7-hydroxycoumarin, was determined fluo...More data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+4nMAssay Description:Inhibitory concentration value against human cytochrome P-450 2D6More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+5nMAssay Description:Inhibitory concentration value against human cytochrome P-450 2B6More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+5nMpH: 7.5Assay Description:To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+5nMAssay Description:To gain insight into the selectivity of the synthetic compounds, nicotine, nicotine related alkaloids and nicotine metabolites for inhibition of othe...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibitory concentration value against human cytochrome P-450 3A4More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+5nMpH: 7.5Assay Description:To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio...More data for this Ligand-Target Pair
Affinity DataAssay Description:The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr...More data for this Ligand-Target Pair