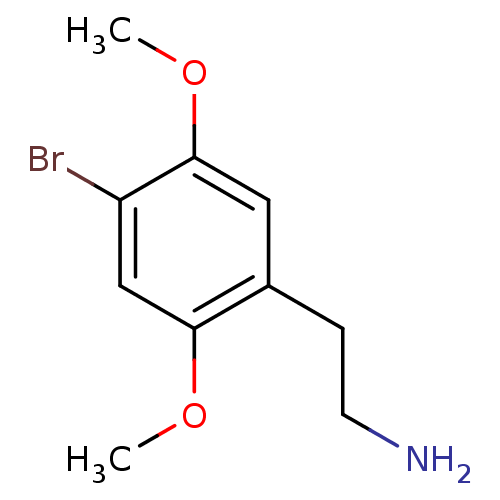
BDBM50005267 2,5-dimethoxy-4-bromophenethylamine::2-(4-Bromo-2,5-dimethoxy-phenyl)-ethylamine::2-(4-bromo-2,5-dimethoxyphenyl)ethylamine::CHEMBL292821::US20240166618, Compound 88
SMILES COc1cc(CCN)c(OC)cc1Br
InChI Key InChIKey=YMHOBZXQZVXHBM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 66 hits for monomerid = 50005267
Found 66 hits for monomerid = 50005267
Affinity DataEC50: 0.0311nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataEC50: 0.264nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.880nMAssay Description:Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]DOI.More data for this Ligand-Target Pair
Affinity DataEC50: 1.89nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataEC50: 1.93nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 6.31nMAssay Description:5-HT2A Receptor Radioligand Binding. Affinity of the test compounds for the 5-HT2A receptor was determined in radioligand binding experiments with [3...More data for this Ligand-Target Pair
Affinity DataKi: 8.25nMAssay Description:5-HT2A Receptor Radioligand Binding. Affinity of the test compounds for the 5-HT2A receptor was determined in radioligand binding experiments with [3...More data for this Ligand-Target Pair
Affinity DataKi: 8.25nMAssay Description:The binding affinities of disclosed compounds at the ketanserin binding site of the 5-HT2A receptor were determined in radioligand binding experiment...More data for this Ligand-Target Pair
Affinity DataKi: 13.5nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]ketanserin from NIH3T3 cells stably expressing rat 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 26.3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 27nMAssay Description:Activity at rat 5HT2A receptor expressed in NIH3T3 cells assessed as stimulation of phospholipase C-mediated IP productionMore data for this Ligand-Target Pair
Affinity DataKi: 27.6nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2 receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using [3H]ketanserin as...More data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Affinity against 5-hydroxytryptamine 2A receptor (K) labeled with [3H]ketanserin.More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Affinity against 5-hydroxytryptamine 2C receptor in J1 cells transfected with the rat 5-HT2C gene labeled with [3H]mesulergine.More data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Compound was tested for binding affinity towards 5-HT1C (5-HT1C) receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using...More data for this Ligand-Target Pair
Affinity DataKi: 89.5nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 103nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 104nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Displacement of [3H]mesulergine from A9 cells stably expressing rat 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 241nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 309nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 665nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 822nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 2.16E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.37E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 7.12E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 7.19E+3nMAssay Description:Agonist activity at human trace amine associated receptor 1 expressed in RD-HGA16 CHO-K1 cells coexpressed with Galpha16 protein assessed as internal...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1/Adenosine receptor A1(Human)
University of Oklahoma
University of Oklahoma
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair