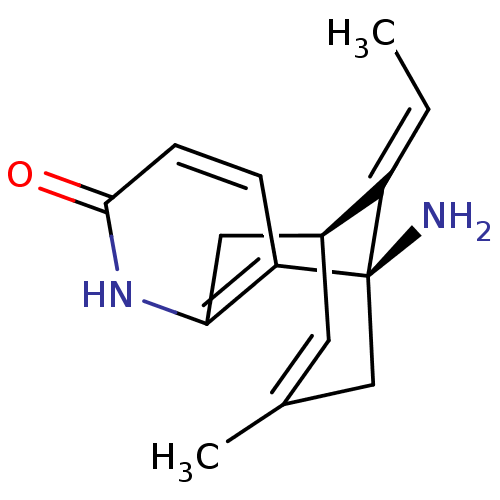
BDBM50199522 (+)-huperzine A::(+-)-HA::(-)-1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one(Huperzine A)::(-)-huperazine A::(-)-huperzine A::(-)1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one( (-)-Huperzine A)::(1R,9R)-1-Amino-13-eth-(E)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::(R)-1-Amino-13-eth-(E)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::(R)-1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::(huperazine A)(-)-1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::1-Amino-13-eth-(E)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::1-Amino-13-eth-(Z)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one::1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one((+/-)-Huperzine A)::1-Amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one((-)-Huperzine A)::1-amino-13-ethylidene-11-methyl-6-azatricyclo[7.3.1.0~2,7~]trideca-2(7),3,10-trien-5-one::CHEMBL395280::HUPERAINE A::Hyperazzine A
SMILES C/C=C/1\[C@@H]2CC3=C([C@]1(CC(=C2)C)N)C=CC(=O)N3
InChI Key InChIKey=ZRJBHWIHUMBLCN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 44 hits for monomerid = 50199522
Found 44 hits for monomerid = 50199522
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
East China University of Science and Technology
Curated by ChEMBL
 3D Structure (crystal)
3D Structure (crystal)