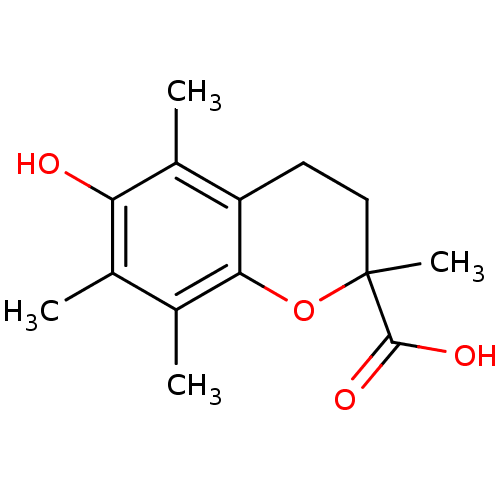
BDBM50359629 TROLOX
SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(O)=O
InChI Key InChIKey=GLEVLJDDWXEYCO-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50359629
Found 9 hits for monomerid = 50359629
TargetProstaglandin G/H synthase 1(Human)
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
TargetProstaglandin G/H synthase 2(Human)
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
TargetProstaglandin G/H synthase 2(Human)
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Affinity DataIC50: 1.31E+4nMAssay Description:Anti-oxidant activity in DPPH radical scavenging assay; n=3-4More data for this Ligand-Target Pair
Affinity DataIC50: 1.86E+5nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min...More data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(White button mushroom)
Kyung Hee University Skin Biotechnology Center
Curated by ChEMBL
Kyung Hee University Skin Biotechnology Center
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of mushroom tyrosinase activity using L-tyrosin as substrate after 20 minsMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Human)
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of Xanthine oxidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins...More data for this Ligand-Target Pair
TargetDehydrogenase/reductase SDR family member 9(Human)
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Institute For Natural Product Research and Infection Biology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of 3alphaHSDMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+6nMpH: 8.0Assay Description:AChE enzymatic activity was measured using an adaptation of the method previously described [Ingkaninan et al., J. Ethnopharmacol., 89:261-264]; 98 &...More data for this Ligand-Target Pair