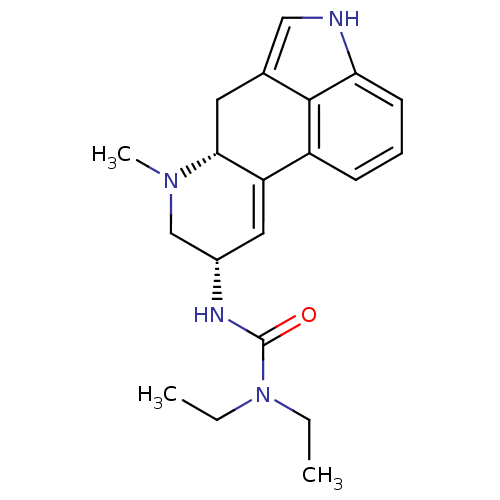
BDBM50056445 1,1-diethyl-3-[(8alpha)-6-methyl-9,10-didehydroergolin-8-yl]urea::CHEMBL157138::N'-((8alpha)-9,10-Didehydro-6-methylergolin-8-yl)-N,N-diethylurea::US20240279226, Example 12::lisuride::lisuride, (S)
SMILES CCN(CC)C(=O)N[C@@H]1CN([C@@H]2Cc3c[nH]c4c3c(ccc4)C2=C1)C
InChI Key InChIKey=BKRGVLQUQGGVSM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 73 hits for monomerid = 50056445
Found 73 hits for monomerid = 50056445
Affinity DataKi: 0.300nMAssay Description:Binding affinity to D2 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKd: 0.300nMAssay Description:In vitro affinity at mutant D2 receptor (S194A) in C6 (glioma) cell membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 0.340nMAssay Description:Binding affinity to dopamine D1 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 0.340nMAssay Description:Binding affinity to dopamine D2 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity towards Serotonin 5-hydroxytryptamine 1A receptor by displacement of [3H]-(+)-8-OH-DPAT.More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKd: 0.400nMAssay Description:In vitro affinity at mutant D2 receptor (S197A) in C6 (glioma) cell membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity towards Dopamine receptor D2 by displacement of [3H]U-86170.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Binding affinity to 5HT1A receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKd: 0.600nMAssay Description:In vitro affinity at wild type Dopamine receptor D2 on C6 (glioma) cell membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Displacement of [125]iodosulpride from human recombinant dopamine D2L receptor expressed in CHO cells after 30 minsMore data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Affinity towards Dopamine receptor D2More data for this Ligand-Target Pair
Affinity DataIC50: 0.970nMAssay Description:Compounds of the present application bind to the 5-HT2 receptor subtypes in the following assays: Compounds of the invention are tested on 5-HT2A and...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]-5HT from human 5-HT2B receptor expressed in CHO-K1 cells assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Binding affinity to D3 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Binding affinity towards Dopamine receptor D3 by displacement of [3H](+)-7-OH-DPAT.More data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to alpha2A adrenergic receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKd: 2.30nMAssay Description:In vitro affinity at mutant D2 receptor (S194A) in C6 (glioma) cell membranes.More data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.20nMAssay Description:Binding affinity to D4 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Binding affinity to 5HT2A (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 6.80nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKd: 10nMAssay Description:Binding affinity for 5-hydroxytryptamine 1D receptor in piglet caudate using [3H]5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 24.6nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Compounds of the present application bind to the 5-HT2 receptor subtypes in the following assays: Compounds of the invention are tested on 5-HT2A and...More data for this Ligand-Target Pair
Affinity DataKi: 35.3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 44.3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Binding affinity to alpha1A adrenergic receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Binding affinity to D1 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 77nMAssay Description:Affinity towards Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 77nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 89nMAssay Description:Agonist activity at recombinant human D4 receptor expressed in CHOK1 cells assessed as induction of beta arrestin2 recruitment measured after 30 mins...More data for this Ligand-Target Pair
Affinity DataKi: 114nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 136nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 203nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 251nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 343nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKd: 501nMAssay Description:Binding affinity for 5-hydroxytryptamine 1A receptor in piglet hippocampus using [3H]8-OH-DPATMore data for this Ligand-Target Pair
Affinity DataKd: >5.01E+3nMAssay Description:Binding affinity for 5-hydroxytryptamine 2 receptor in rat cortex using [3H]- ketanserinMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)