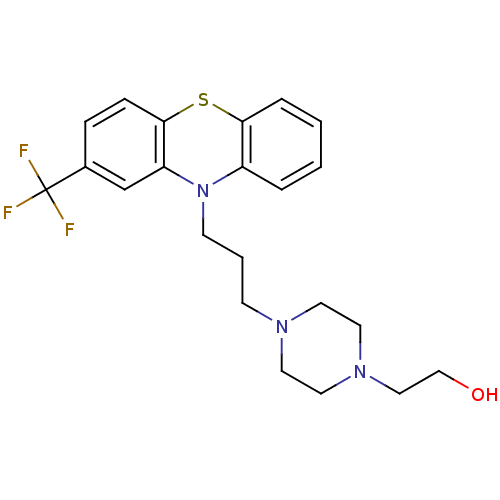
BDBM78433 2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]propyl]-1-piperazinyl]ethanol;hydrochloride::2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]piperazin-1-yl]ethanol;hydrochloride::2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]propyl]piperazino]ethanol;hydrochloride::FLUPHENAZINE::FLUPHENAZINE HYDROCHLORIDE::MLS001076508::SMR000058411::cid_3372::cid_6602611::med.21724, Compound 17
SMILES OCCN1CCN(CCCN2c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1
InChI Key InChIKey=PLDUPXSUYLZYBN-UHFFFAOYSA-N
Data 95 KI 21 IC50 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB 119 hits for monomerid = 78433
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
BDBM78433 (2-[4-[3-[2-(trifluoromethyl)phenothiazin-10-yl]pro...)
Copy SMILES Copy InChI
Displayed 1 to 50 (of 119 total ) | Next Last
 Found 119 hits for monomerid = 78433
Found 119 hits for monomerid = 78433