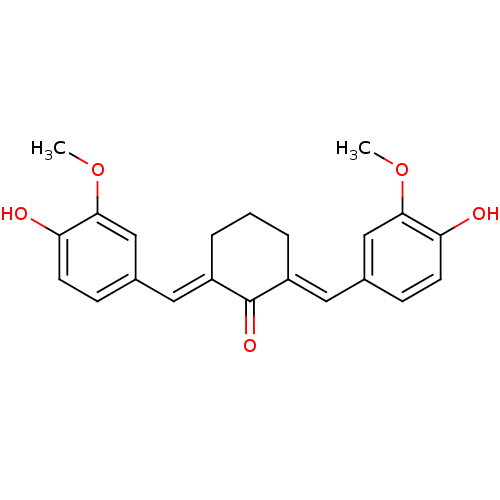
BDBM50067033 (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone::2,6-Bis((3-methoxy-4-hydroxyphenyl)methylene)-cyclohexanone::2,6-Bis-(4-hydroxy-3-methoxy-benzylidene)-cyclohexanone::2,6-Bis-[1-(4-hydroxy-3-methoxy-phenyl)-meth-(E)-ylidene]-cyclohexanone::2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone::CHEMBL17205::Cyclovalone::US9187397, 38a::cid_1550234
SMILES COc1cc(\C=C2/CCC\C(=C/c3ccc(O)c(OC)c3)C2=O)ccc1O
InChI Key InChIKey=DHKKONBXGAAFTB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50067033
Found 10 hits for monomerid = 50067033
TargetNucleotide-binding oligomerization domain-containing protein 2(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.75E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 4.94E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:The NF-kB activities of curcumin and analogs were determined by a cellular firefly luciferase assay. This assay utilized a commercially available cel...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+3nMAssay Description:Curcumin is a known inhibitor of the AP-1 activation cascade. Therefore, modification of the structure of curcumin could lead to analogs with enhance...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:The NF-kB activities of curcumin and analogs were determined by a cellular firefly luciferase assay. This assay utilized a commercially available cel...More data for this Ligand-Target Pair
Affinity DataIC50: 1.11E+5nMAssay Description:Inhibition of GST-p300 HAT assessed as histone acetylationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Compound concentration required to reduce HIV-1 Integrase 3'-processing activity by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against HIV-1 Integrase (HIV-1-IN)More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of tissue factor in Homo sapiens (human) THP1-cells using factor 10a chromogenic substrate assessed as inhibition of LPS-iduced procoagula...More data for this Ligand-Target Pair