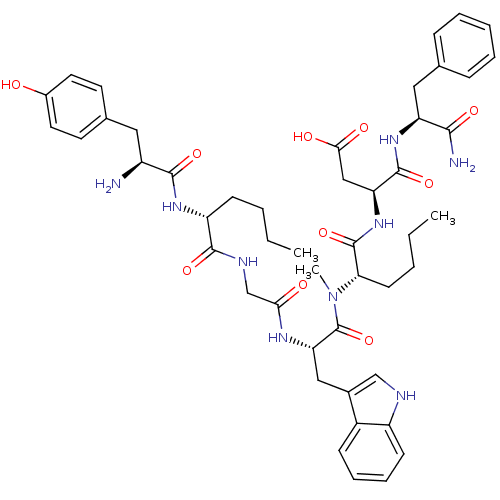
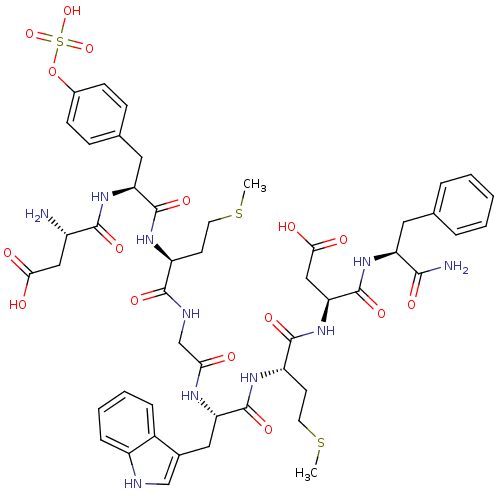
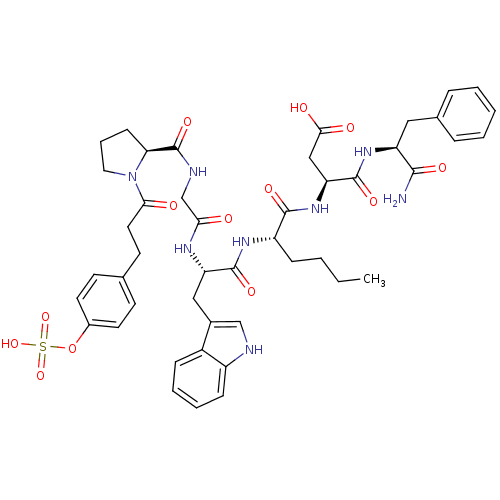
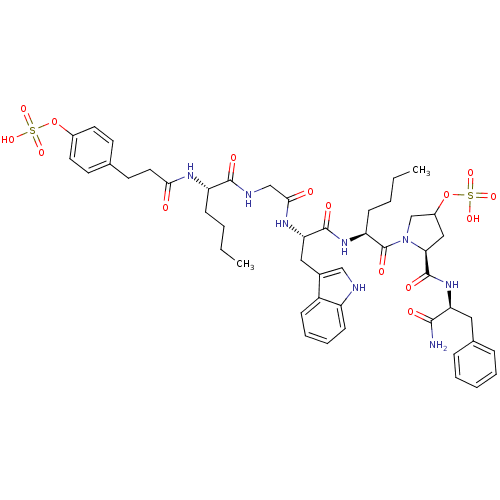
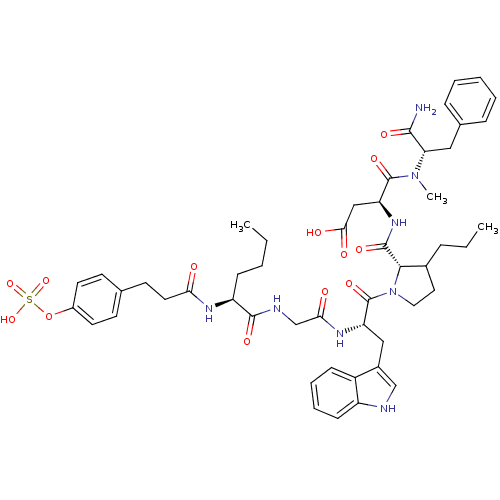
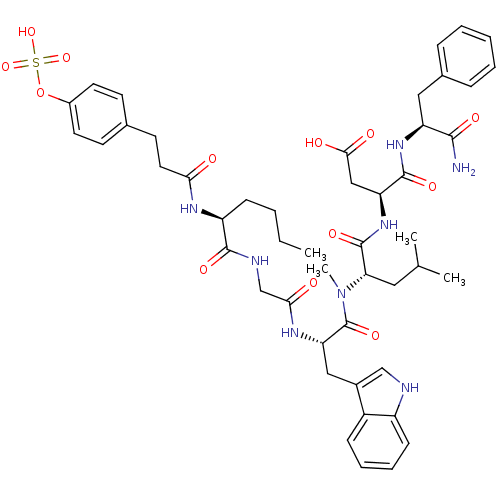
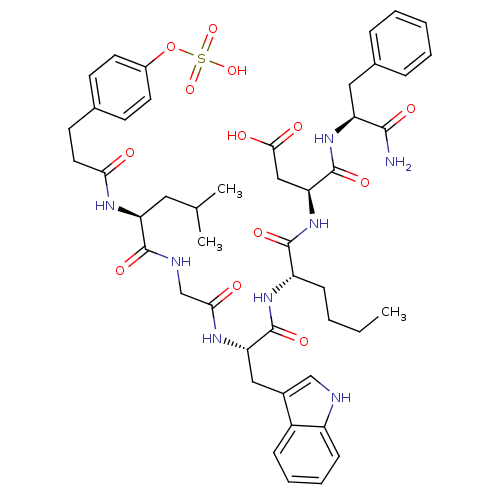
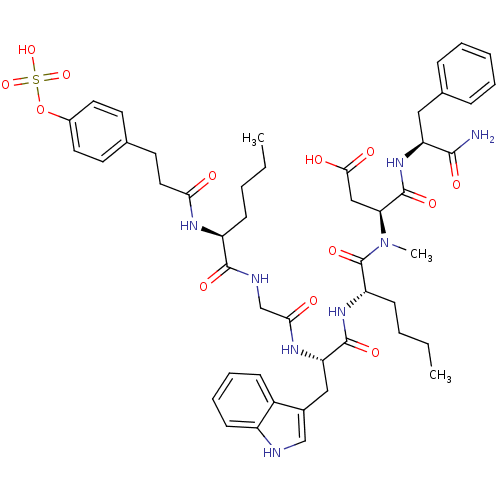
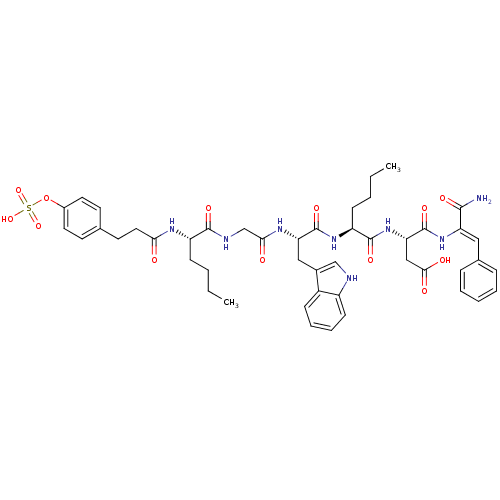
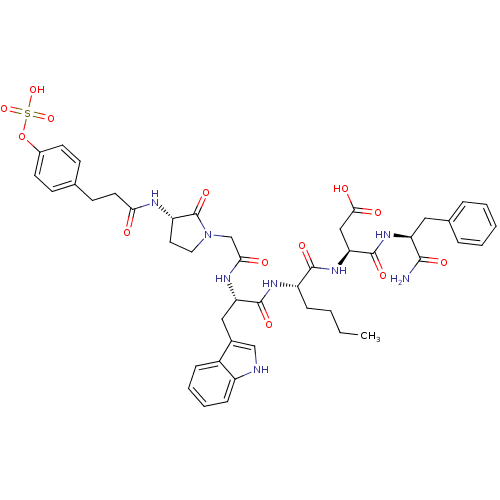
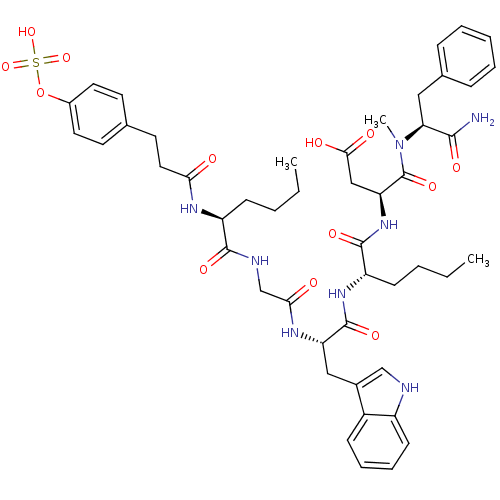
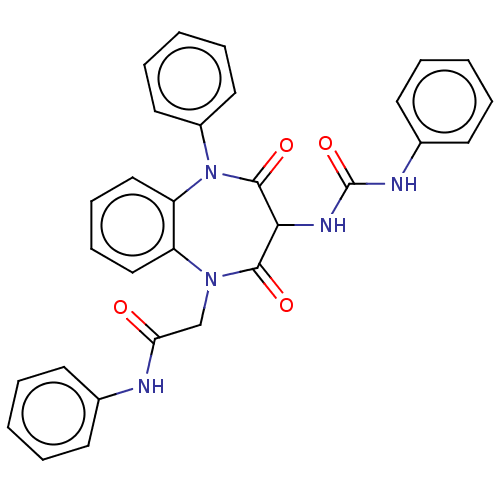
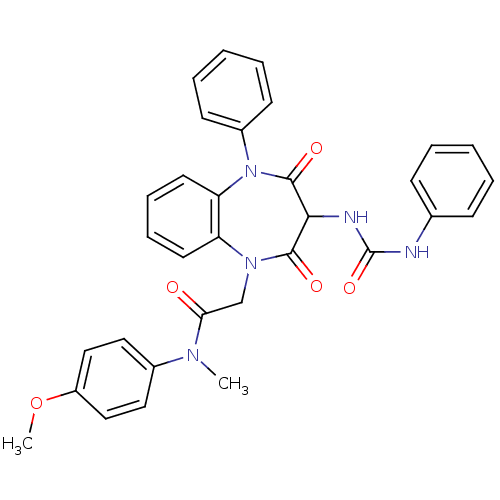
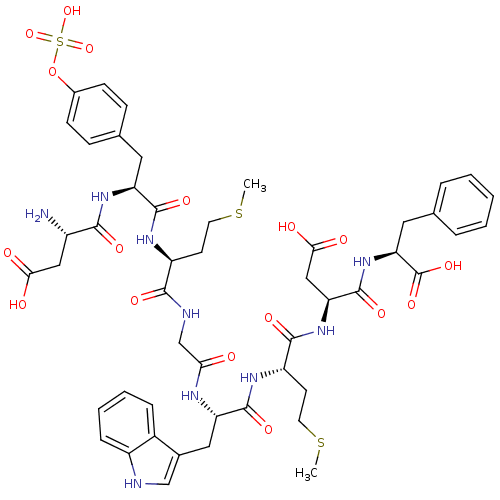
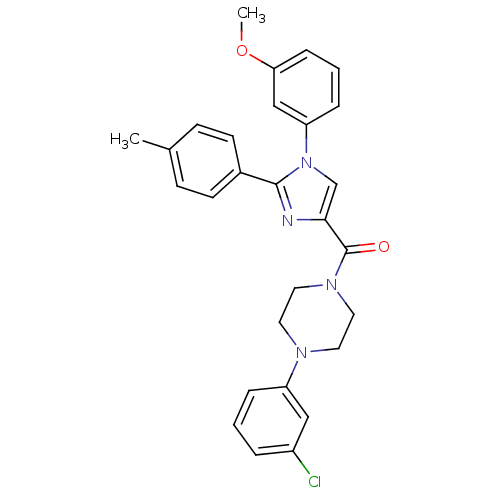
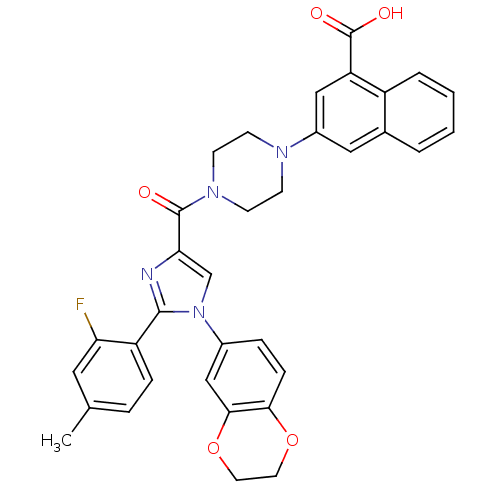
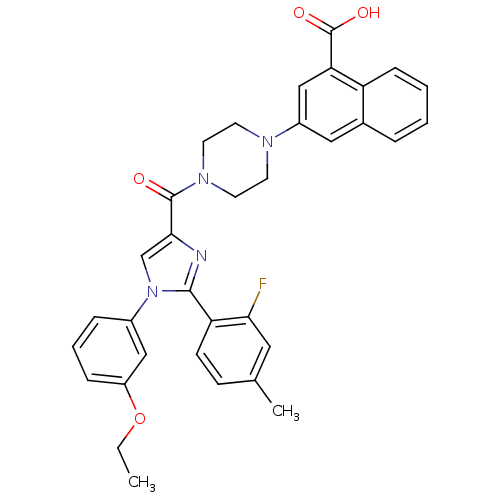
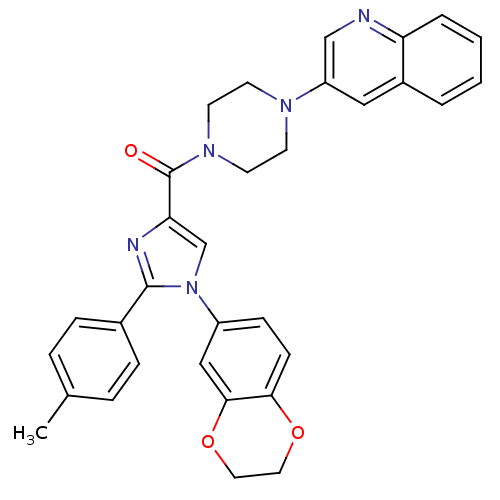
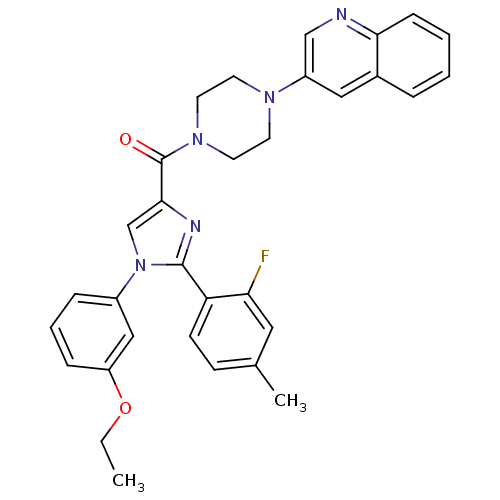
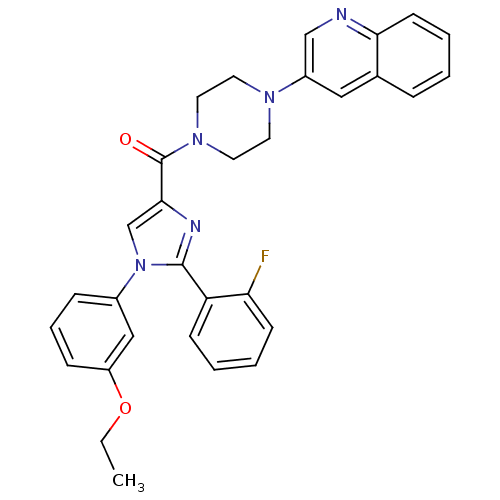
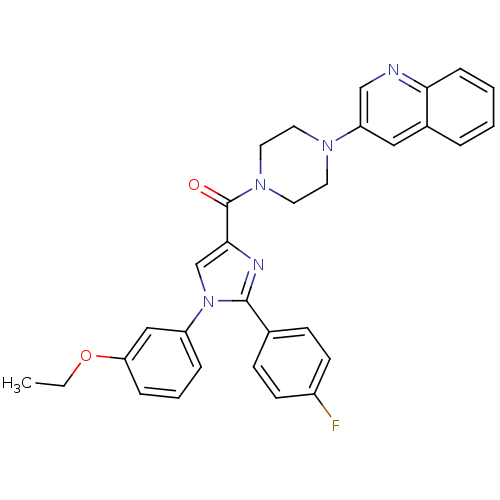
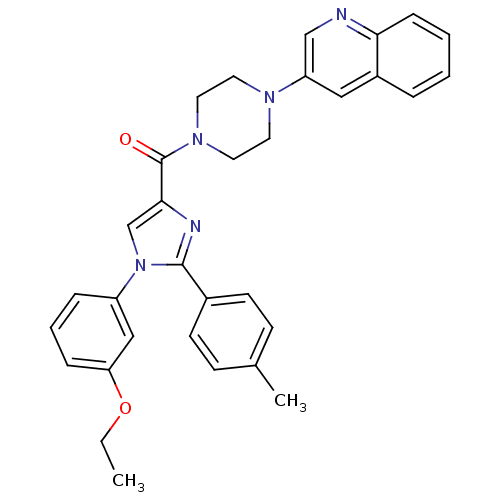
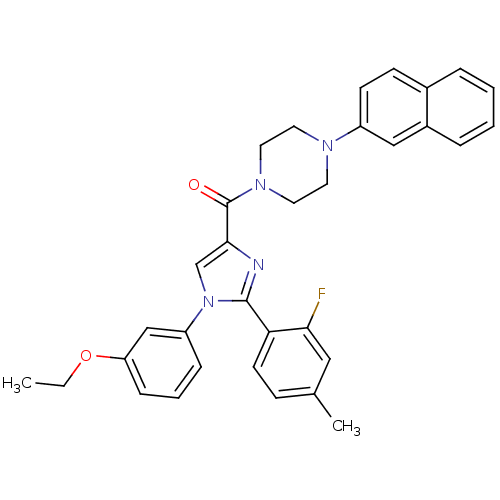
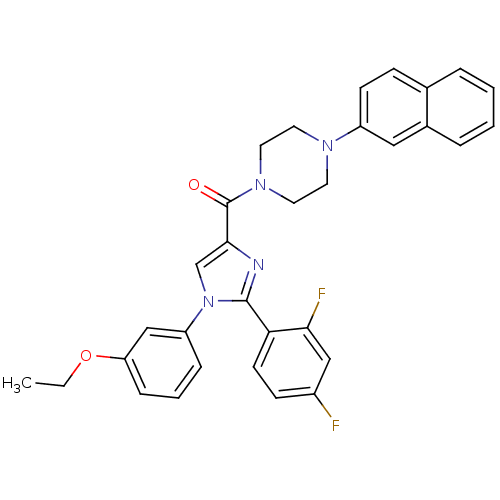
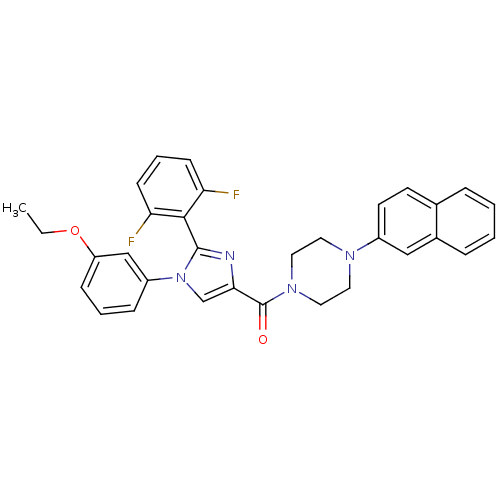
Affinity DataAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
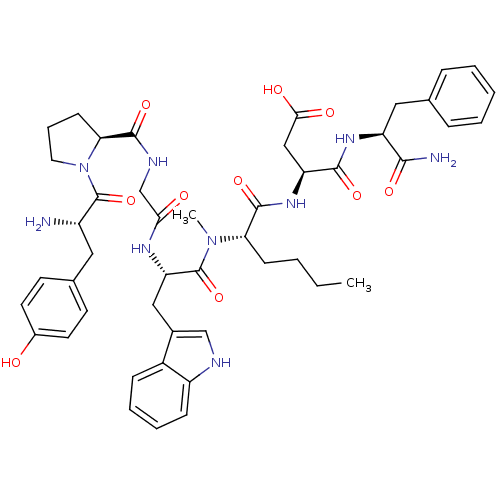
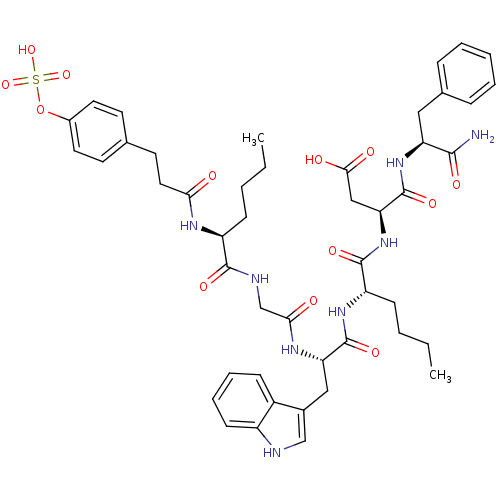
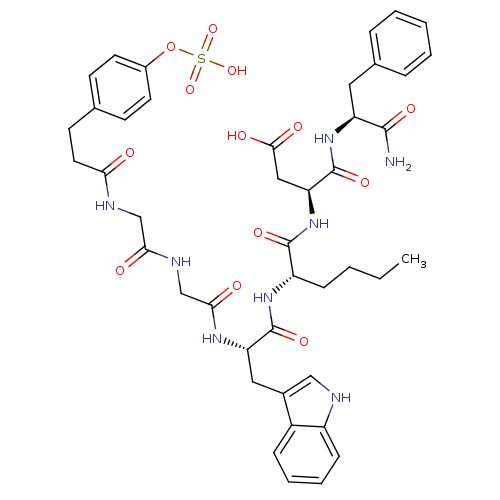
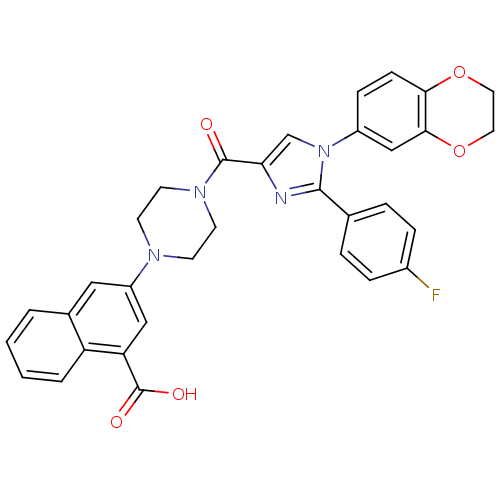
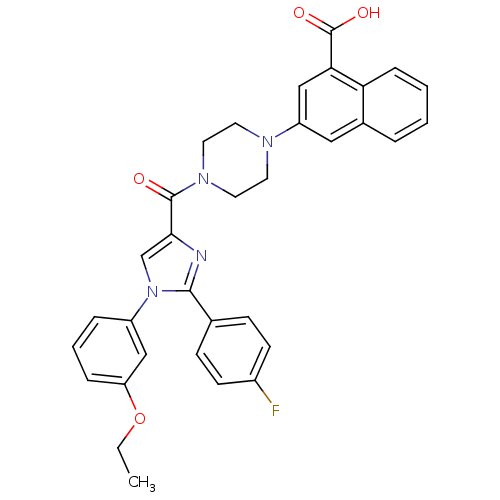
Affinity DataAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
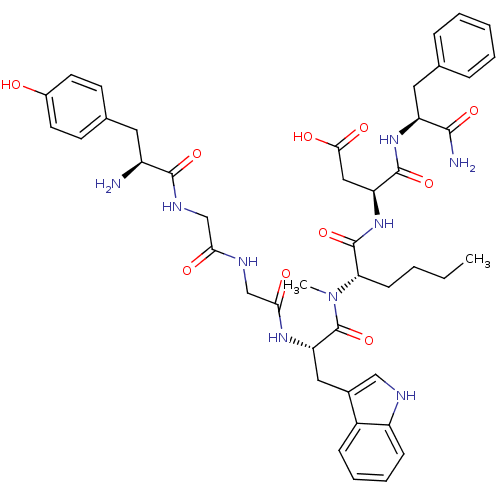
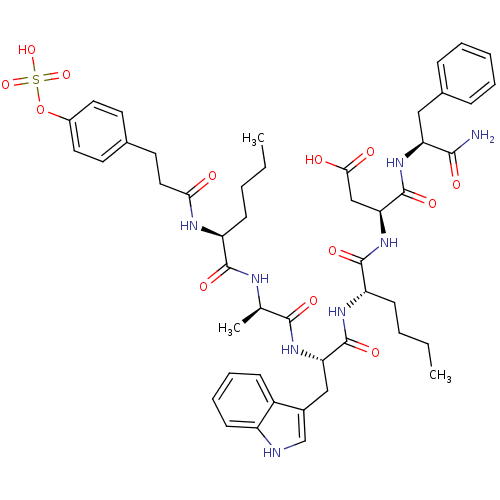
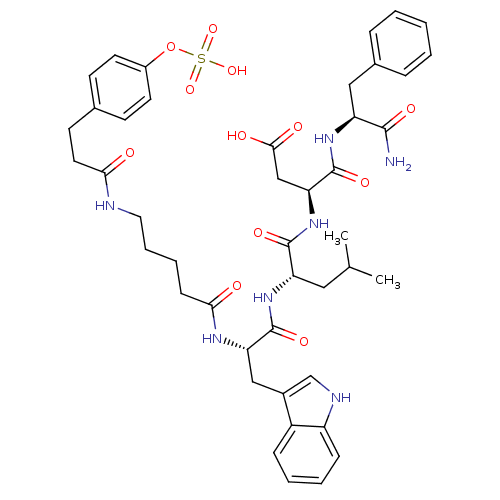
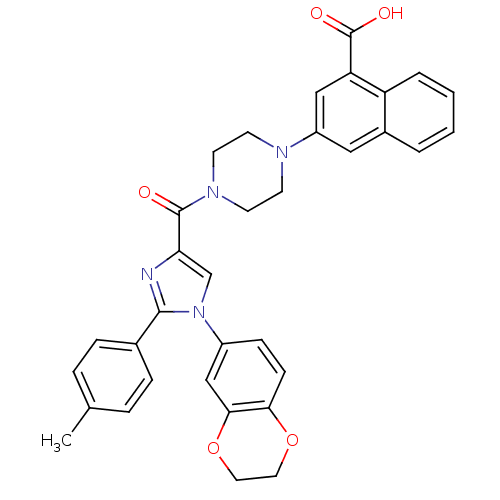
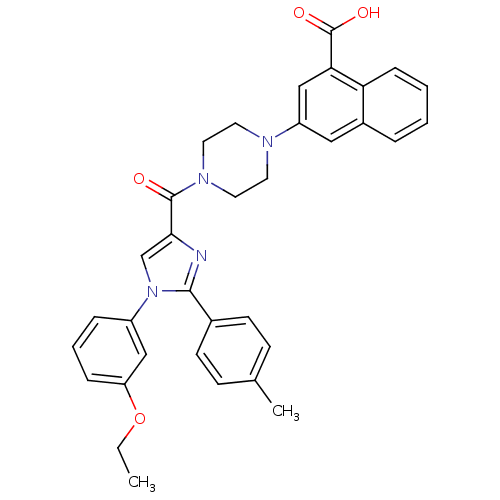
Affinity DataAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
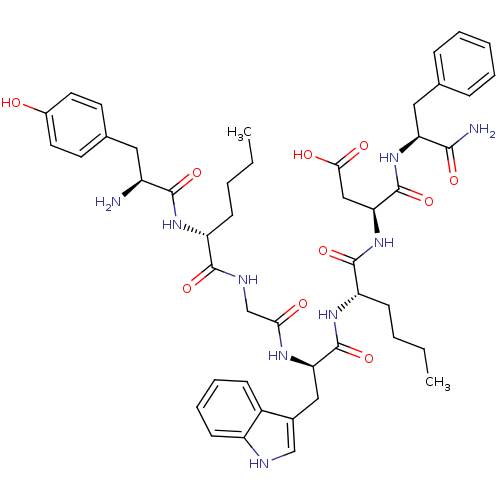
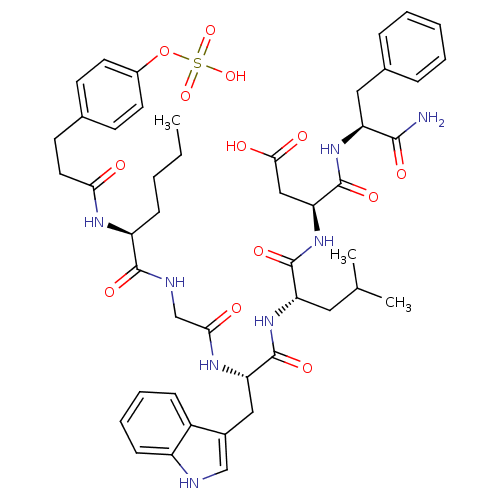
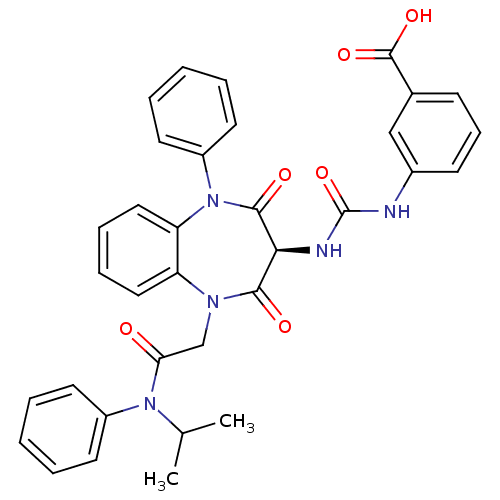
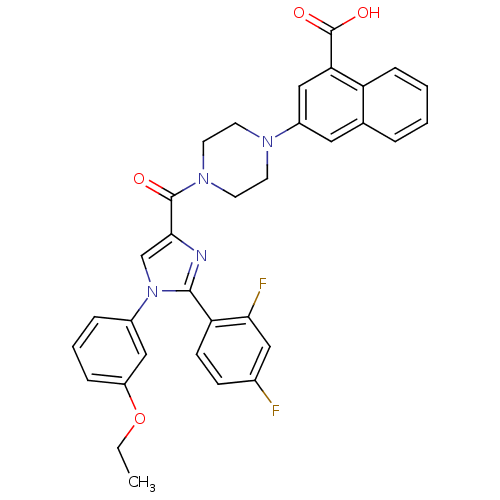
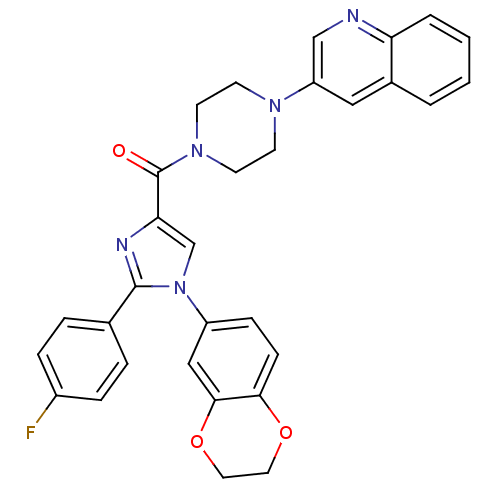
Affinity DataAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataKd: 1.90nM EC50: 28nMAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.700nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 6.90nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 1.30nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 15nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.990nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 50nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 3.70nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.640nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.100nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 2.5nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 71nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 0.320nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 3.70nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataEC50: 140nMAssay Description:Evaluated in vitro for maximal stimulatory activity for amylase release relative to CCK-8 in guinea pig tissueMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0130nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.01 nM (Rvb = 7 to 14 ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0100nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.1 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataEC50: 0.00200nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.316 nM (Rvb = 7 to 14...More data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1 nM (Rvb = 7 to 14 pM)More data for this Ligand-Target Pair
Affinity DataEC50: 0.0190nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0200nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0140nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0510nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0390nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1000 nM (Rvb = 7 to 14 ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.120nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1000 nM (Rvb = 7 to 14 ...More data for this Ligand-Target Pair
Affinity DataEC50: 90nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as stimulation of intracellular calcium responsesMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+3nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as stimulation of intracellular calcium responsesMore data for this Ligand-Target Pair
Affinity DataEC50: 0.800nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as stimulation of intracellular calcium responsesMore data for this Ligand-Target Pair
Affinity DataEC50: 28nMAssay Description:Activity at human CCK1 receptor expressed in HEK293 cells assessed as level of [3H]inositol produced relative to controlMore data for this Ligand-Target Pair
Affinity DataEC50: 74nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0770nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0930nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0530nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0560nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0480nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0940nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.260nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.120nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.130nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.530nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.25nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.730nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 4.40nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair