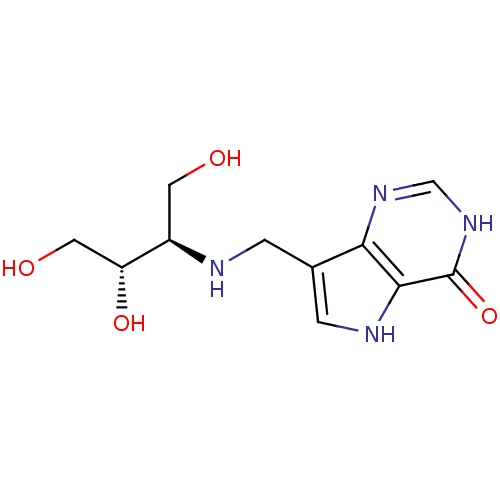
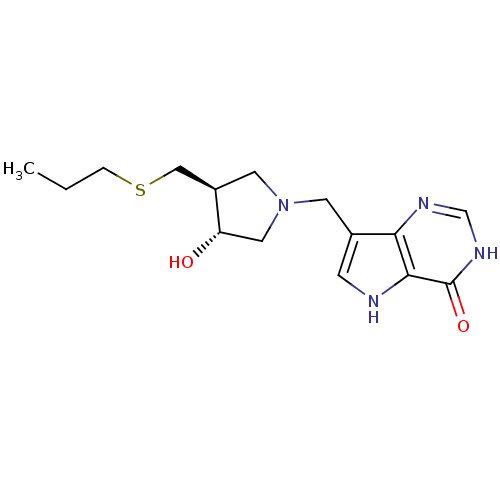
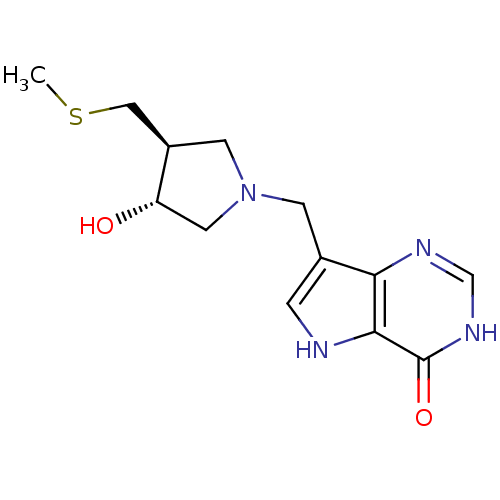
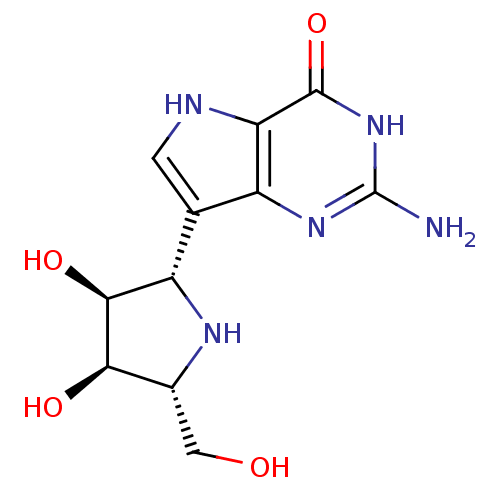
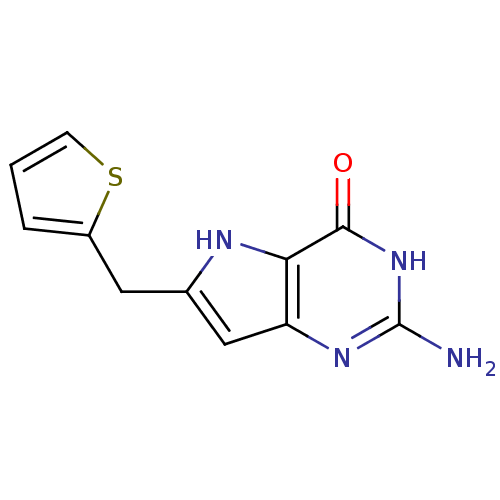
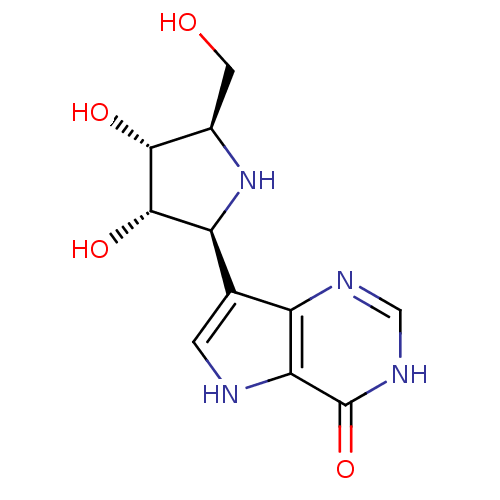
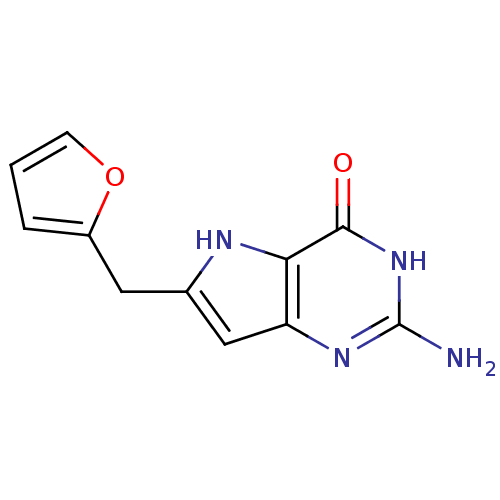
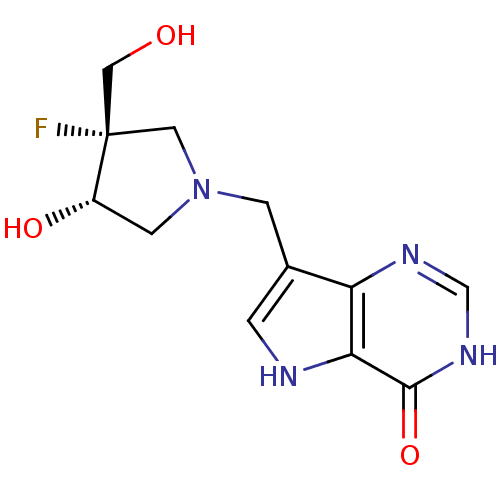
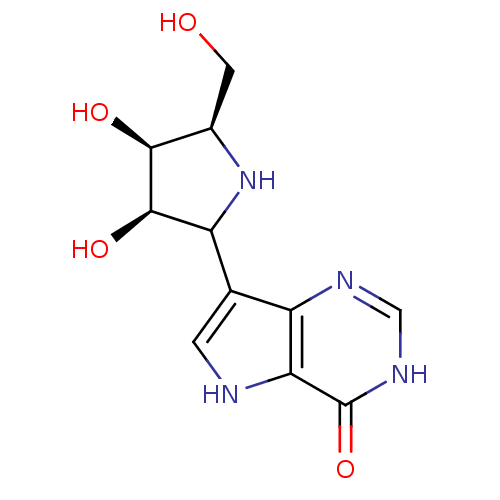
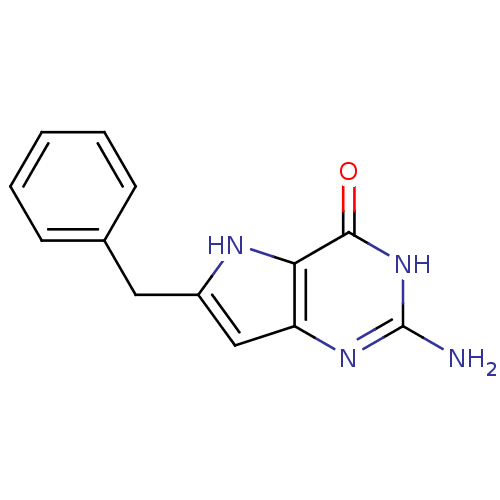
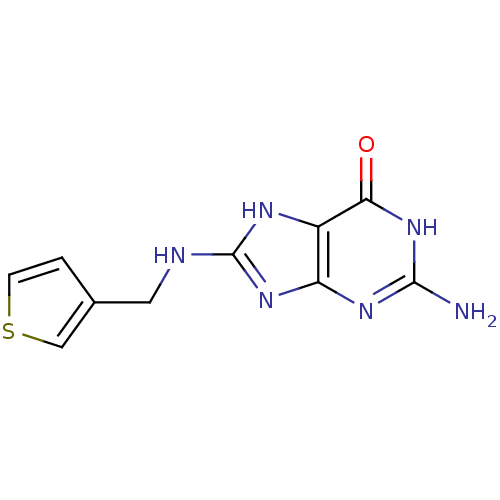
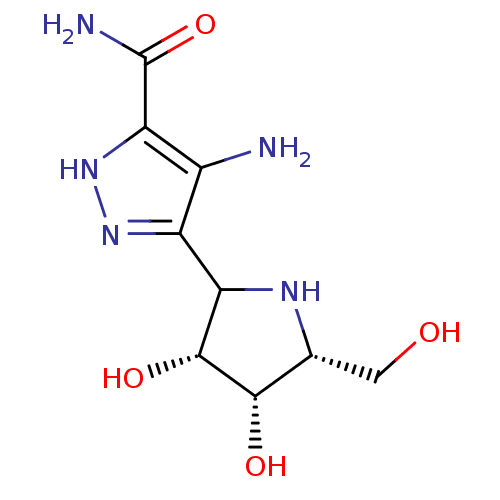
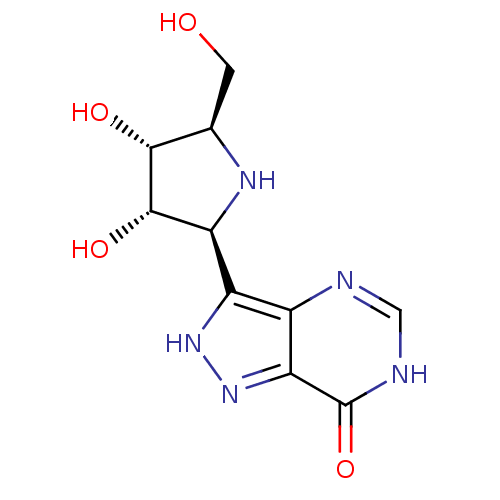
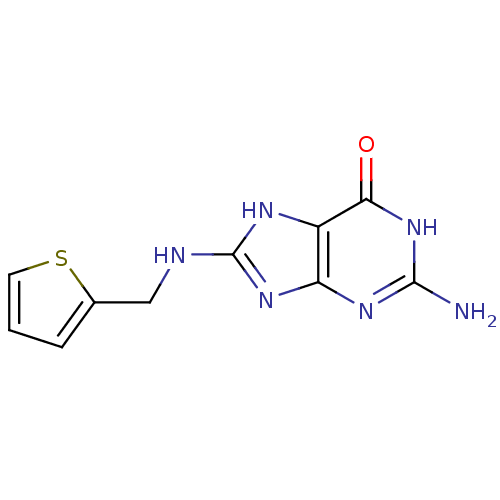
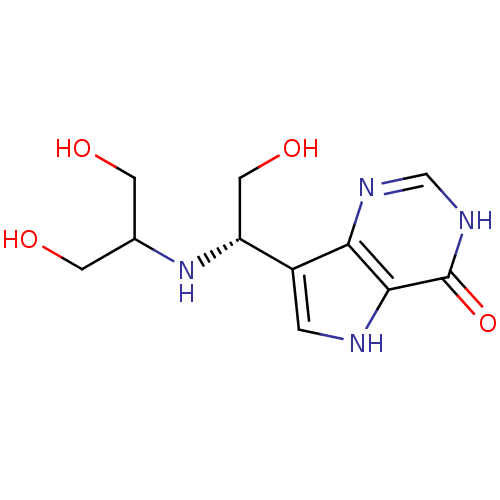
Affinity DataKi: 0.00200nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00300nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00500nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00520nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.00680nMAssay Description:Dissociation constant against Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKi: 0.00700nMAssay Description:Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ...More data for this Ligand-Target Pair
Affinity DataKi: 0.00700nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKd: 0.00850nMAssay Description:Binding affinity to human PNPMore data for this Ligand-Target Pair
Affinity DataKd: 0.00860nMT: 2°CAssay Description:The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements...More data for this Ligand-Target Pair
Affinity DataKi: 0.00900nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00900nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00980nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0107nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0110nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0160nMAssay Description:Inhibition of human His-tagged PNP assessed as equilibrium dissociation constant by measuring reduction in uric acid formation by spectrophotometric ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0160nMAssay Description:Dissociation constant against Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKi: 0.0196nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0290nMAssay Description:Inhibitory activity of compound against human purine nucleoside phosphorylase (PNP)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 0.0420nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0420nMAssay Description:Dissociation constant against Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKi: 0.0420nMAssay Description:Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 0.0560nMAssay Description:Inhibitory activity of compound against human purine nucleoside phosphorylase (PNP)More data for this Ligand-Target Pair
Affinity DataKi: 0.0560nMAssay Description:Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 0.0560nMAssay Description:Dissociation constant against Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKd: 0.0560nMAssay Description:Inhibition of human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0560nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0570nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 0.0579nMAssay Description:Equilibrium binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKd: 0.0580nMAssay Description:Binding affinity to human PNPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of human recombinant PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.0660nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0660nMAssay Description:Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0670nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 0.0700nMAssay Description:Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0720nMAssay Description:Inhibitory activity of compound against human purine nucleoside phosphorylase (PNP)More data for this Ligand-Target Pair
Affinity DataIC50: 0.0850nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0850nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 0.0960nMAssay Description:Inhibitory activity of compound against human purine nucleoside phosphorylase (PNP)More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.104nMAssay Description:Inhibitory activity of compound against human purine nucleoside phosphorylase (PNP)More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKi: 0.117nMAssay Description:Initial binding affinity to wild type human PNPMore data for this Ligand-Target Pair
Affinity DataKd: 0.156nMAssay Description:Inhibition assay using human PNP.More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphateMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of human His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation by measuring reduction in uric acid formatio...More data for this Ligand-Target Pair
Affinity DataKi: 0.163nMAssay Description:Binding affinity towards Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Dissociation constant against Human Purine Nucleoside Phosphorylase was reportedMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibition of human PNP by xanthine-oxidase coupled assayMore data for this Ligand-Target Pair
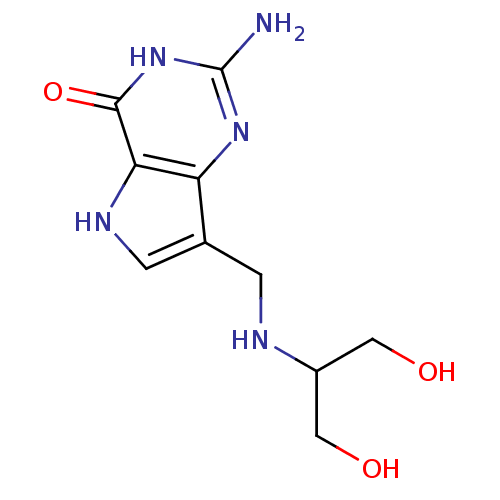
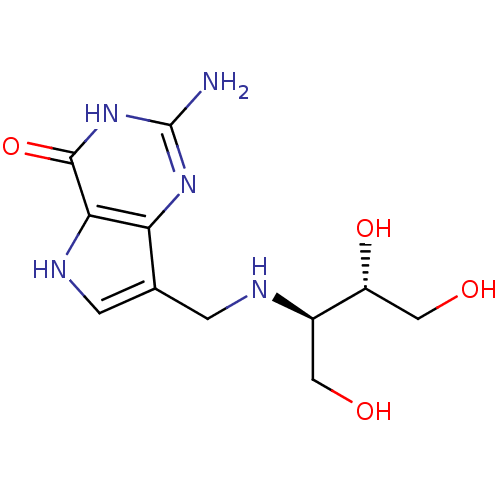
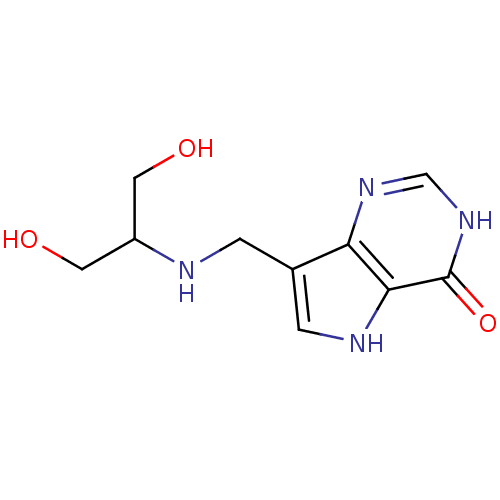
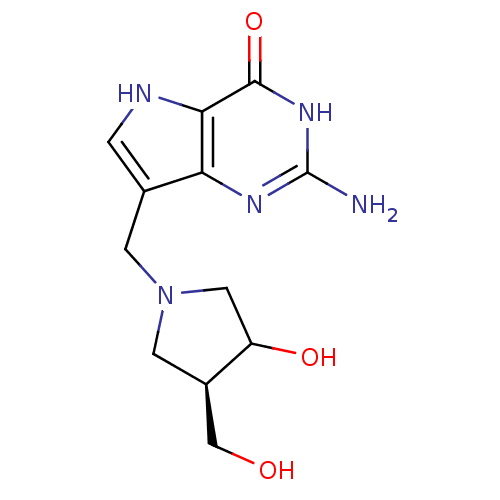
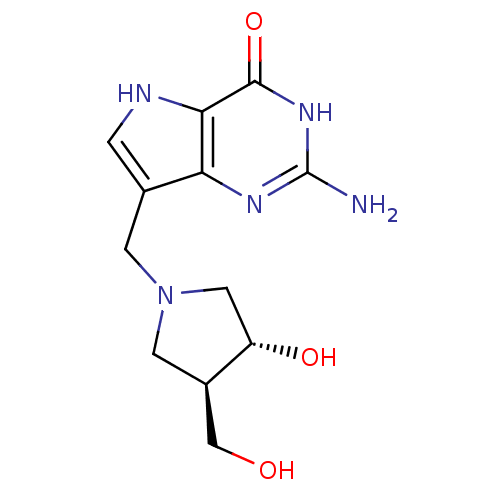
 3D Structure (crystal)
3D Structure (crystal)