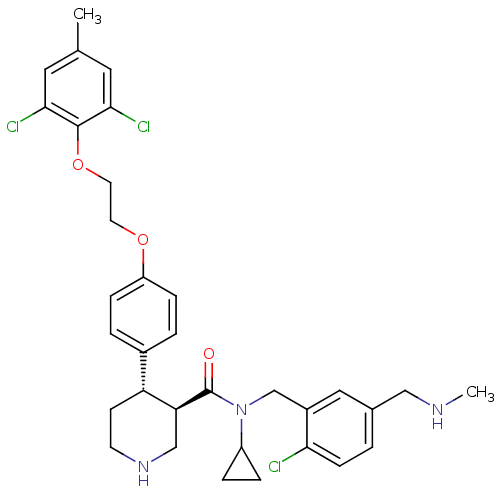
BDBM50328850 (3R,4S)-N-(2-chloro-5-((methylamino)methyl)benzyl)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-methylphenoxy)ethoxy)phenyl)piperidine-3-carboxamide::CHEMBL1269674
SMILES CNCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1
InChI Key InChIKey=VKUUNUNXIVLQGO-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50328850
Found 4 hits for monomerid = 50328850
Affinity DataIC50: 1.39nMAssay Description:Inhibition of renin in bufferMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of renin in bufferMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of CYP3A4 using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP3A4 using testosterone as substrateMore data for this Ligand-Target Pair